Abstract
Chronic lymphocytic leukemia (CLL) is the most common leukemia in Western Countries. This pathology is characterized by an accumulation of monoclonal, non-functional and mature CD5+ CD19+ leukemic B-cells (CLL cells) in lymph nodes, peripheral blood and bone marrow. Despite a high resistance to the in vivo apoptosis, CLL cells die spontaneously in vitro due to a lack in ex vivo conditions of sustaining cells and factors from their microenvironment such as stroma cells (Lagneaux L et al, Blood. 1998; 91:2387-2396), follicular dendritic cells or Nurse-Like-Cells (NLC) (Burger JA et al, Blood. 2000; 96:2655-2663).
NLC are derived from CD14+ cells in contact with CLL cells in vitro (Tsukada N et al, Blood. 2002; 99:1030-1037) and were found in lymph nodes of CLL patients (Ysebaert L et al, Leuk Lymphoma. 2011; 52:1404-1406). NLC were shown to have a Tumour Associated Macrophages phenotype and gene expression profile. These cells have been first described to be essential for in vitro CLL cells survival partially through the production of soluble factors such as CXCL12 (Burger JA et al, Blood. 2000; 96:2655-2663), CCL3 and CCL4 (Burger JA et al, Blood. 2009; 113:3050-3058). Thus, other mechanisms are required for CLL cells survival. Indeed, we showed that contact of CLL cells with NLC was necessary to protect CLL cells from the in vitro apoptosis. We then investigated the mechanism of these interactions at a molecular level. We also determined their influences on the in vitro CLL cells survival and on the NLC-induced chemoresistance.
We observed close and strong interactions evaluated by the measurement of trogocytosis from NLC to CLL cells. Trogocytosis is an active phenomenon with transfer of membrane fragments from one cell to another. We showed that NLC/CLL cells trogocytosis is dependant to actin polymerization and SRC phosphorylation.
To find possible couples of molecules involved in this contact, we compared different transcriptomic data from NLC, monocyte, CLL cells and B lymphocytes. We highlighted potential couples of molecules and confirmed their expression on CLL cells and NLC by flow cytometry analysis. Finally, we obtained 3 couples probably implicated: Lymphocyte Function-Associated Antigen 3 (LFA-3)/CD2, Platelet/Endothelial Cell Adhesion Molecule 1 (PECAM1)/CD38 and Intercellular Adhesion Molecule 1 (ICAM-1)/LFA-1.
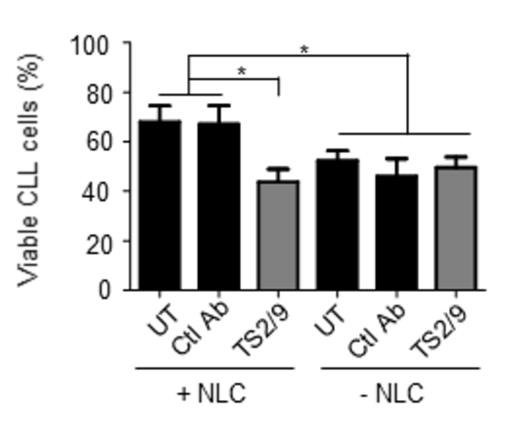
Antibody blocking strategies revealed that LFA-3, which is up-regulated in CLL cells compared to healthy donors B lymphocytes, was necessary for the interaction between CLL cells/NLC when PECAM1, ICAM-1 and their co-partners were not essential (figure 1). Furthermore, this contact, through LFA-3, induced Akt phosphorylation but not ERK1/2 phosphorylation in CLL cells. Finally, we showed that LFA-3 and its receptor CD2 are necessary to the rescue of CLL cells by NLC (figure 2).
To go further, we tested the chemoprotective effect of NLC on CLL cells. We showed that NLC slightly protect CLL cells against bendamustin but not against rituximab, dasatinib or ibrutinib. We hypothesized that the contact through LFA-3 could be involved in this chemoresistance. However, we did not observed a significant effect of the combination of bendamustin and LFA-3-blocking compared to bendamustin alone suggesting that this chemoprotection of CLL cells by NLC involved another pathway.
Altogether, our results indicate that overexpression of LFA-3 by CLL cells and its critical implication in the interaction with NLC might be a new therapeutic target in CLL to disturb the interaction of CLL cells with their microenvironment.
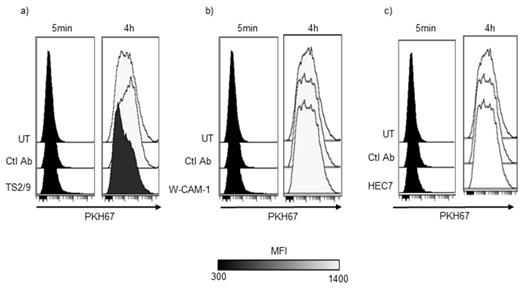
LFA-3 blocking but not ICAM-1 and PECAM1 decrease trogocytosis from NLC to CLL cells. a) Representative overlay of an experiment of trogocytosis from NLC to CLL cells treated or not by a blocking antibody anti-LFA-3. b) Representative overlay of an experiment of trogocytosis from NLC to CLL cells treated or not by a blocking antibody anti-ICAM-1. c) Representative overlay of an experiment of trogocytosis from NLC to CLL cells treated or not by a blocking antibody anti-PECAM1.
LFA-3 blocking but not ICAM-1 and PECAM1 decrease trogocytosis from NLC to CLL cells. a) Representative overlay of an experiment of trogocytosis from NLC to CLL cells treated or not by a blocking antibody anti-LFA-3. b) Representative overlay of an experiment of trogocytosis from NLC to CLL cells treated or not by a blocking antibody anti-ICAM-1. c) Representative overlay of an experiment of trogocytosis from NLC to CLL cells treated or not by a blocking antibody anti-PECAM1.
LFA-3 is critical for the survival of CLL cells in contact with NLC.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal