Abstract
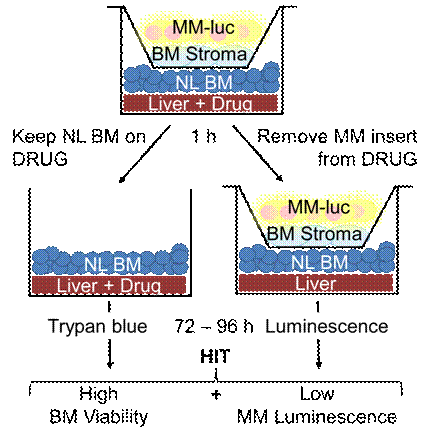
Taking a mechanistically unbiased approach to the discovery of new myeloma drugs we developed a three organ system assay that selected anti-myeloma compounds from a primary 30,000 small molecule ATP-based screen in one sandwich setup for tolerability by normal bone marrow, stability towards liver enzymes, and activity in the context of cell barriers, bone marrow stromal support, and short, kidney-clearance-like exposure (fig.1). The most promising compound (CCF642) emerging from this test had IC50s around 500 nM in all 8 MM cell lines tested including a proteasome inhibitor refractory line while the IC50 was not reached in 5 healthy bone marrow samples at doses up to 6750 nM. Similar sensitivity as in MM was seen in 7 lymphoma cell lines. Investigation of its mechanism of action revealed that CCF642 immediately stimulates protein ubiquitination which is followed by degradation of major myeloma survival factors (c-MYC, IRF4, NF-κB) and apoptosis, while normal bone marrow cells increase ubiquitination to a much lesser degree and only transiently without undergoing cell death. Experiments with an active biotinylated analog showed that it preferentially enters myeloma cells with cytoplasmic accumulation and over 10-fold lower entry into normal bone marrow. Immunoblots revealed that it becomes covalently attached to proteins in a time- and distribution pattern that parallels ubiquitination responses. In vitro, it bound to ubiquitin activating enzyme UBA1 at the active site cysteine in a similarly stable and reversible way as ubiquitin. Selective alkylation of cysteine sulfhydryl groups inhibited binding of biotinylated CCF642 to UBA1, while addition of ATP and ubiquitin led to its dissociation from UBA1. CCF642 may therefore at least in part act as a small molecule ubiquitin analog. Accordingly CCF642 binding to target proteins of UBA1 containing HeLa fraction II was increased by addition of ATP and blocked in the presence of EDTA. Injected twice a week IP at 30mg/kg it suppressed systemically engrafted luciferase expressing 5TGM1 mouse myeloma cells in syngeneic mice comparable to bortezomib given at the MTD for this mouse strain (0.5mg/kg SC twice a week) with equal prolongation of survival. Results support use of our sandwich assay to select myeloma drug candidates for in vivo testing and reveal a new UBA1 interacting small molecule ubiquitination enhancer with promise for clinical translation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal