IMPORTANCE: Gastrointestinal bleeding is one of the most common sites of bleeding complicated by anticoagulant therapy. Patients with anticoagulant-associated gastrointestinal (GI) bleeding are at risk of both thromboembolic events after anticoagulant interruption and recurrent bleeding following anticoagulant resumption.
OBJECTIVE: To determine the risk of thromboembolism, recurrent GI bleeding and mortality for long-term anticoagulated patients who experienced GI bleeding.
DATA SOURCES: We searched MEDLINE, EMBASE and CENTRAL from inception-July 2014, conferences abstracts (January 2006-July 2014) and www.clinicaltrials.gov up to the last week of January 2014 with no language restriction.
STUDY SELECTION: Randomized controlled trials and cohort studies
DATA EXTRACTION AND SYNTHESIS: Two reviewers independently performed study selection, data extraction and study quality assessment.
MAIN OUTCOMES AND MEASURES: Selected outcomes were thromboembolic events, recurrent gastrointestinal bleeding and all-cause mortality.
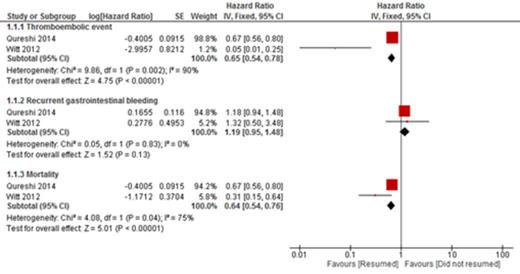
RESULTS: A total of 6 studies were included in the qualitative analysis and 2 studies in the quantitative analysis. Thromboembolic events occurred in 92 of 984 patients (9.34%) who resumed warfarin and in 147 of 895 (16.4%) patients who did not. The resumption of warfarin was associated with a significant reduction in thromboembolic event (HR 0.65 [95% CI, 0.54 to 0.78], p < 0.001, I2=90%) Recurrent GI bleeding occurred in 101 of 954 (10.59%) patients who restarted warfarin and in 40 of 895 (4.47%) patients who did not. There was no statistically significant increase in recurrent GI bleeding for patients who restarted warfarin compared to those who did not (HR 1.19 [95% CI, 0.95 to 1.48], p = 0.13, I2 = 0%). Death occurred in 203 of 984 (20.63%) patients who resumed warfarin and 316 of 896 (35.27%) patients who did not resume warfarin. Resumption of warfarin was associated with significant reduction in mortality (HR 0.64 [95% CI, 0.54 to 76], p <0.01, I2 = 75%).
CONCLUSIONS AND RELEVANCE: This meta-analysis demonstrates that resumption of warfarin following interruption due to GI bleeding is associated with a reduction in thromboembolic events and mortality without a significantly increased risk of recurrent GI bleeding.
Forrest plot of the estimate effect of thromboembolic event, recurrent gastrointestinal bleeding and mortality in patient who resumed anticoagulant versus patients who did no resume
Forrest plot of the estimate effect of thromboembolic event, recurrent gastrointestinal bleeding and mortality in patient who resumed anticoagulant versus patients who did no resume
Crowther:Leo Pharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; CSL Behring: Speakers Bureau; Boehriniger Ingelheim: Consultancy; Shire: Speakers Bureau; Celgene: Speakers Bureau; Bayer: Speakers Bureau; Asahi Kasai: Membership on an entity's Board of Directors or advisory committees; Portola: Membership on an entity's Board of Directors or advisory committees; Viropharma: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal