Abstract
Background: Platelets are required for effective treatment of severe hemorrhage. Standard-of-care storage is at room temperature (RT), but leads to a storage lesion characterized by loss of hemostatic function and increased risk of bacterial contamination. Refrigeration (4C) mitigates adverse effects; however, it results in pre-activation or priming, which may be suggestive of prothrombotic tendencies. We previously showed that 4C-stored platelets (4C-PLT) retain responses to physiological inhibitors comparable to those of fresh platelets (FR-PLT) in two static aggregation assays. To more closely mimic in-vivo conditions, we tested adhesive response in a microfluidic environment under physiologic high-shear flow. We hypothesized that 4C-PLT display superior adhesion compared to standard-of-care (RT-PLT) and that platelet hemostatic inhibition due to prostacyclin and nitric oxide (NO) would be similar to fresh.
Methods: Apheresis platelets (AP) collected from 4 healthy donors were stored for 5 days at RT (22-24°C) or 4C (1-6°C). Additional whole blood was collected to obtain red blood cells (RBCs). Platelet samples were assayed on Day 1 (fresh) and Day 5 (RT-PLT and 4C-PLT) in the presence or absence of prostacyclin (10 nM Prostaglandin I2, PGI2) or an NO donor (50 uM S-Nitrosoglutathione, GSNO). Bioflux plates (Fluxion) were coated with 100 µg/ml type-1 collagen. Prior to perfusion, platelets were stained with calcein-AM (300x103PLT/ul) and RBCs were added to a hematocrit of 40%. Samples were perfused through the collagen-coated wells at an arterial shear rate of 720s-1, and compared to bovine serum album (BSA)-coated channels as a control to assess nonspecific binding. A fluorescence microscope acquired images every 30 sec for 6 min. Data were reported as fluorescence intensity units (FIU) and surface coverage (SC%) measured with Bioflux Montage (MetaMorph) software. Data were analyzed using two-way ANOVA and a post hoc Tukey test for multiple comparisons. Significance was p<0.05.
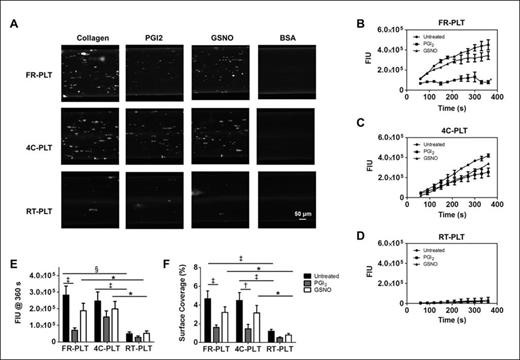
Results: The perfusion images illustrate that 4C-PLT adhesion was similar to FR-PLT and demonstrably better than RT-PLT (Fig.1A). Measured fluorescence confirmed visual findings (Fig. 1B-E) and demonstrated that RT-PLT adhesion was considerably attenuated. Cold-stored platelet average SC% after 6 min of perfusion was similar to fresh (4C-PLT: 4.5±0.8%; FR-PLT: 4.7±0.8%; p=0.86; Figure 1F), but that of RT-PLT was significantly decreased (RT-PLT: 1.2±0.2%; p<0.001; Figure 1F). The addition of PGI2 to all groups reduced adhesion to collagen-coated surfaces under high shear (FR-PLT: 1.6±0.3%; p<0.001; 4C-PLT: 1.5±0.5%; p<0.01; RT-PLT: 0.5±0.1%; p≥0.05). GSNO treatment was similar, although reductions were not statistically significant (FR-PLT: 3.2±0.6%; 4C-PLT: 3.2±0.8%; RT-PLT: 0.8±0.2%; p≥0.05).
Conclusion: Although 4C-stored platelets show increased levels of activation compared to fresh, they retain comparable responses to physiological inhibitors under shear flow. Furthermore, standard-of-care RT-stored platelets are unable to adhere to collagen under flow conditions. These data suggest that 4C platelets, although primed and hemostatically more active, may respond to homeostatic signals in vivo and may not pose a risk of promoting unregulated clot formation. RT-PLT function is significantly reduced and did not recover activity in this physiologically relevant model. Testing 4C-PLT in a small animal model of trauma could further elucidate these findings.
Platelet adhesion under high shear with and without inhibitors. (A) Micrographs of FR-PLT, 4C-PLT, and RT-PLT samples are shown for a single donor after 6 minutes of perfusion. Representative fluorescence intensity unit (FIU) traces are shown for (B) FR-PLT, (C) 4C-PLT, and (D) RT-PLT (Untreated •, PGI2 ■, and GSNO ▲). Data for (E) FIU (n=4) and (F) SC% (n=4) after 360 s are mean ± SEM (Untreated = black, PGI2= gray, and GSNO = white). The micrographs were cropped and enlarged; however image size proportionality was maintained between groups and timepoints. * p<0.05, † p<0.01, and ‡ p<0.001, and § p<0.0001
Platelet adhesion under high shear with and without inhibitors. (A) Micrographs of FR-PLT, 4C-PLT, and RT-PLT samples are shown for a single donor after 6 minutes of perfusion. Representative fluorescence intensity unit (FIU) traces are shown for (B) FR-PLT, (C) 4C-PLT, and (D) RT-PLT (Untreated •, PGI2 ■, and GSNO ▲). Data for (E) FIU (n=4) and (F) SC% (n=4) after 360 s are mean ± SEM (Untreated = black, PGI2= gray, and GSNO = white). The micrographs were cropped and enlarged; however image size proportionality was maintained between groups and timepoints. * p<0.05, † p<0.01, and ‡ p<0.001, and § p<0.0001
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal