Abstract
Introduction: MicroRNA (miRNA) is a class of non-coding small RNA (~22 nucleotides), which regulates post-transcriptional gene expression through binding to complementary sites of target messenger RNA. Recent studies have revealed that miRNA plays important roles in oncogenesis mainly by regulating oncogenes or tumor suppressor genes. As miRNA exists not only within cells but also in peripheral blood, circulating miRNA can be an easily obtainable cancer biomarker. Furthermore, circulating miRNA, which exists within exosomes or as a complex with particular proteins such as argonaute2, is a suitable and convenient sample for handling because of its stability. Based on these findings, this study planned to pursue the possibility that circulating miRNA could be used for the early diagnosis of diffuse large B-cell lymphoma (DLBCL).
Materials and Methods: This study was performed with the permission of our IRB. Expression levels of mature miRNA (both the 5p and 3p strands) were evaluated using different types of materials, namely serum, exosome-enriched serum, and formalin-fixed paraffin-embedded (FFPE) tissue. Serum samples were obtained from patients with newly diagnosed DLBCL (n=33) or healthy volunteers (n=22) at our institution between 2012 and 2014. The exosome-enriched samples were obtained from serum using a total exosome isolation reagent (Invitrogen, CA). The FFPE biopsy samples were obtained from DLBCL lesions, mainly from lymph nodes (n=22), or from reactive hyperplastic lymph nodes (n=6). Based on the results of previous reports, ten miRNAs (the 5p and 3p strands of miR-15a, miR-21, miR-155, miR-181a, and miR-210) were selected as candidate biomarkers. Expression levels of miRNA were analyzed by the quantitative Real-Time PCR method, and were normalized to hsa-miR-24-3p. Diagnostic accuracy was evaluated using receiver operating characteristics (ROC) curve analysis.
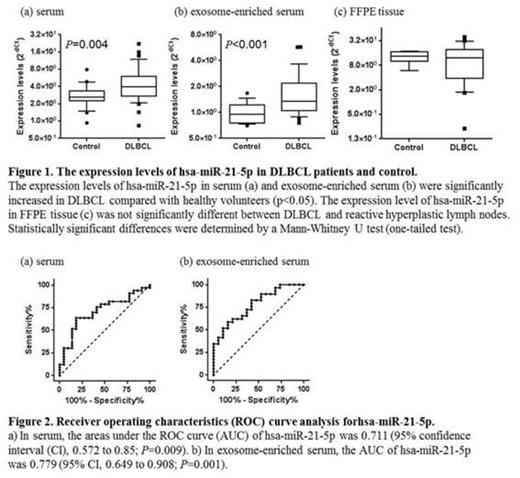
Results and Discussion: In serum samples, the expression levels of hsa-miR-15a-3p, hsa-miR-21-5p, hsa-miR-181a-5p, and hsa-miR-210-5p were significantly different between DLBCL patients and healthy volunteers (p<0.05). ROC curve analysis revealed that hsa-miR-15a-3p and hsa-miR-21-5p were valuable biomarkers for differentiating patients with DLBCL from healthy volunteers with an area under the ROC curve (AUC) of 0.676 (p=0.029) and 0.711 (p=0.009), respectively. However, substantial percentages of false-positive and false-negative samples were also observed. To improve diagnostic accuracy, we next analyzed the expression levels of miRNAs in exosome-enriched serum samples. Among the candidate miRNAs, expression levels of hsa-miR-15a-3p, hsa-miR-21-5p, and hsa-miR-181a-5p were significantly different between DLBCL and healthy volunteer in exosome-enriched serum samples (p<0.05). ROC curve analysis revealed that hsa-miR-15a-3p, hsa-miR-21-5p, and hsa-miR-181a-5p were valuable biomarkers with AUCs of 0.714 (p=0.033), 0.779 (p=0.001), and 0.672 (p=0.047), respectively. Contrary to these results, expression levels of these miRNAs in FFPE were not significantly different between DLBCL and reactive lymph nodes. We thus consider that some miRNAs in serum or exosome-enriched serum might be useful in the differential diagnosis of DLBCL. These miRNAs seem to be produced outside of the lymphoma tissue. To use miRNAs as an accurate and sensitive diagnostic biomarker, it is necessary to first identify lymphoma cell-derived miRNA in blood.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal