Abstract
Despite its high efficacy on CML, long-term exposure to nilotinib, a second generation tyrosine kinase inhibitor (TKI2), has been reported to increase the onset of arterial cardiovascular events (CVE) in chronic phase (CP) CML patients (pts), especially in pts with cardiovascular risk factors. However, some pts without any cardiovascular risk factors may experience arterial thrombotic events and the pathogenesis of this phenomenon remains obscure. Homocystein (HC) is a key sulphured amino-acid derived from methionin, independently associated with increased frequencies of thrombo-embolic events, early arteriosclerosis and increased cardiovascular mortality (Nygard NEJM 1997). In addition, in vitro, this amino-acid induces the proliferation of smooth muscle cells, endothelial cell dysfunction, increased collagen synthesis and exerts pro-inflammatory rearrangements within the arterial wall.
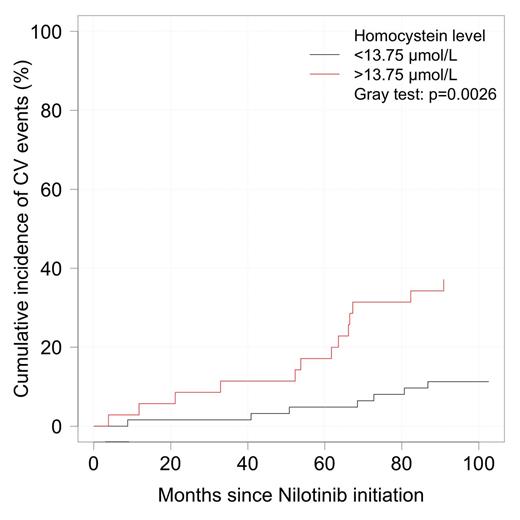
In the present study, we wanted to determine if hyperhomocysteinemia might influence the onset of cardio-vascular events in a series of 114 CP CML pts on nilotinib. We have prospectively analysed in a multicentric study, on-nilotinib cohorts of CP CML pts between September 2011 and July 2014 with standard clinical assessments [height, weight, body mass index (BMI)], onset of any CV events, and basic routine blood metabolic laboratory assessments (fasting glucose, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, glycosylated haemoglobin, homocysteinemia (Biorad kit on HLPC on Summit Dionex system, Thermo Fischer scientific, France), vitamines B9 and B12. All the 114 pts were in CP-CML on nilotinib [43 as first-line, 71 as second-line or more, since a median of 51.5 (3-164) months], 49 were males, with a median age of 51.7 (18-81) at CML diagnosis and 58 (82-20) at nilotinib initiation. Twenty-eight (24.5%) pts presented some CV risk factors prior to nilotinib initiation, and 24 (21%) had an active tobacco abuse at assessment (4 patients unknown). Median weight was 69 (44-149) kg and median BMI was 24.5 (16.5-46) kg/m2 (8 patients >30). Overall, 21 (18.5) pts presented new or worsened pre-existing CVE on nilotinib after a median follow-up of 51.5 months since TKI2 initiation. These CVE occurred after a median of 47 (7.5-82.3) months with a regular increase along the years (Cumulative incidence (CI) 3% at 1 year, 4.12% at 2, 5.15% at 3, 9.30 at 5 and 20.62% at 8.3 years). In total, 3 pts died, 2 from brain stroke, 1 from sudden death in a pt with a history of myocardial infarction. In an univariate analysis we compared our cohort of nilotinib pts to a similar cohort of 17 pts on imatinib as control [median age 56 years since CML diagnosis (p=ns), median duration of imatinib 39 months (p=ns), median weight 70.5 kg (p=ns), BMI 25.9 kg/m2 (p=ns)]. None of these imatinib pts had shown any CVE during follow-up. HC was significantly lower in imatinib-treated pts (p=0.029), in female pts (p=0.018) and increased with age (p<0.001), fasting glucose (p=0.001), glycosylated haemoglobin (p=0.027) were significantly lower too. There was no correlation between HC and cholesterol (total, HDL, LDL). We constructed in the nilotinib cohort a ROC curve to determine the HC cut-off threshold for CVE which was 13.75 mmol/l [AUC=0.68 (95% CI: 0.54-0.82), sensitivity 65%, specificity 71%]. We looked at the CI of CVE according to the HC levels, segregating the pts according to the HC threshold. There was a significant difference (p=0.003) in the incidence of CVE in patients on nilotinib according to the HC level, as shown on figure 1.
Cumulative incidence of CVE on nilotinib according to the level of HC.
The CI of CVE was significantly higher in pts on nilotinib 800 mg daily (38% at latest follow-up) versus pts on ≤600 mg daily, (10%, p=0.005, Gray test). Finally multivariate analysis identified age [p<0.001, HR 1.05 (95% CI 1.02-1.09)], 800 mg daily nilotinib dose [p=0.0049, HR 7.08 (95% CI 1.81-27.6)], and HC [p=0.043, HR 1.11 (95% CI 1.03-1.19)], with an increased risk of CVE on nilotinib.
In conclusion, nilotinib seems to favour CVE in the long-term in CP CML pts, especially in patients with CV risk factors, and these CVE are specifically linked to higher doses of nilotinib and high levels of homocysteinemia. This marker could represent a useful tool to detect pts at risk of arterial CVE on nilotinib. Whether hyperhomocysteinemia is one of the causes of CVE of a consequence of nilotinib treatment remains to be determined.
Michallet:Genzyme: Consultancy; Oseus: Consultancy; BMS: lectures, lectures Other; Novartis: lectures, lectures Other; MSD: lectures Other. Etienne:Novartis: Consultancy; BMS: lectures, lectures Other. Nicolini:Novartis: Consultancy, Honoraria; BMS: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal