Abstract
Background: Primary myelofibrosis (PMF) can have a very heterogeneous clinical course and outcome, rendering prognostic stratification tools a relevant aid for treatment-related decision-making, both at diagnosis and during the progression of the disease. Prognostic categorizations such as the IPSS (International Prognostic Scoring System) (Cervantes et al, Blood 2009) and DIPSS (Dynamic IPSS) (Passamonti et al, Blood 2010) distinguish four risk groups with progressively worsening survival and have the advantage of including clinical and laboratory variables available worldwide [namely age >65 years, presence of constitutional symptoms, hemoglobin (Hb) level <10 g/dL, leukocyte (WBC) count >25 x109/L, and circulating blast cells 1% or greater]. PMF prognostication has been recently supplemented with genetic [e.g., karyotype banding in DIPSS-Plus (Gangat et al, J Clin Oncol 2011)] and molecular (e.g., somatic mutations in ASXL1, SRSF2, IDH1/2, EZH2, CALR) data.
The current challenge in low risk (LR) IPSS/DIPSS is to identify those patients who are at higher risk of progression, and who, possibly, need different therapeutic strategies. Although molecular profiling promisingly seems to identify LR-IPSS/DIPSS-Plus patients with a less favorable outcome in terms of overall survival and risk of leukemic transformation (Vannucchi et al, Leukemia 2013; Tefferi et al, Leukemia 2014), it still remains not ubiquitously available.
Aim: To identify clinical predictors associated with worse outcome in terms of event-free survival (EFS) in a cohort of LR-IPSS PMF patients.
Patients and methods: This study was performed on 125 PMF patients belonging to the LR-IPSS category at diagnosis, i.e. none of the IPSS/DIPSS risk factors present. Patients derived from an update of the original DIPSS cohort now including 483 regularly monitored PMF patients (Table 1). The Institutional Review Board approved the study and the procedures followed were in accordance with the Declaration of Helsinki. Events as defined in the current study included death, leukemic transformation, and shift to a higher risk DIPSS category (i.e., progression to intermediate-1, intermediate-2, or high risk). Standard Kaplan-Meier and Cox regressions were used to assess the impact of covariates on EFS. Age at diagnosis, correlated to EFS by definition, was included in the regression and will not be reported here.
Results: Median EFS for the LR-IPSS PMF patient cohort was 6.37 years (95% CI: 5.05-8.74, Figure 1).The 5- and 10-year cumulative incidences of the single components of the EFS were as follows: 35.2% and 61.5% respectively for DIPSS progression, 1.6% and 1.6% respectively for leukemic transformation, and 4.2% and 5.4% respectively for death. Cox proportional-hazards regression showed that low Hb level and magnitude of splenomegaly and hepatomegaly (all measured at diagnosis and considered as continuous covariates) and platelet (Plt) count at diagnosis <150 ´109/L were significantly associated with EFS besides the expected impact of advanced age.
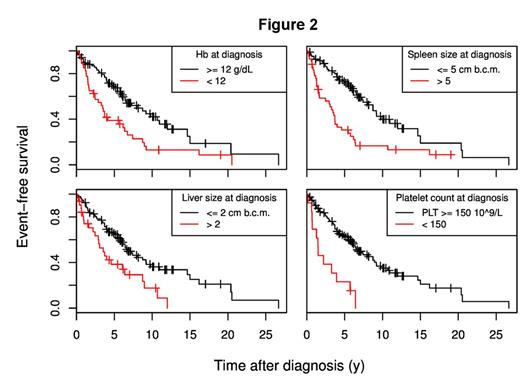
To simplify the interpretation, we discretized the continuous variables; in the resulting model, Hb level <12 g/dL (hazards ratio -HR- 1.67, 95% CI: 1.02-2.72, p=0.040), Plt count <150 ×109/L (HR 2.38, 95% CI: 1.22-4.65, p=0.011), splenomegaly >5 cm below the costal margin (bcm) (HR 1.84, 95% CI: 1.13-2.99, p=0.014), and hepatomegaly >2 cm bcm (HR 2.04, 95% CI: 1.19-3.51, p=0.0097) retained their impact on EFS (Figure 2).
Conclusion: This study indicates that patients with LR-IPSS PMF presenting with Hb levels between 10 and 12 g/dL, or Plt counts <150 ´109/L, or with palpable spleen size >5 cm bcm, or with palpable liver size >2 cm bcm are at higher risk of undergoing DIPSS progression, death or leukemia. The identification of these parameters, available everywhere, can help treatment-related decision-making.
TG and FP equally contributed to this work as senior authors.
Demographics of PMF patients
| Characteristics . | Values . |
|---|---|
| Number of patients | 125 |
| Sex | F: 52 (42%) / M: 73 (58%) |
| Age at diagnosis (years): median, range | 52.0 (18.0-64.0) |
| Hb (g/dL): median, range | 12.5 (10.1-15.8) |
| Hb <12 g/dL | 40 (32%) |
| Plt (x109/L): median, range | 457.0 (47.0-1563.0) |
| Plt <150 x109/L | 13 (10%) |
| WBC (x109/L): median, range | 9.6 (2.2-23.5) |
| Spleen size (cm bcm): median, range | 4.0 (0.0-25.0) [1 NA] |
| Palpable | 93 (74%) |
| >5 cm bcm | 42 (34%) |
| Liver size (cm bcm): median, range | 1.0 (0.0-10.0) |
| Palpable | 64 (51%) |
| >2 cm bcm | 32 (26%) |
| Characteristics . | Values . |
|---|---|
| Number of patients | 125 |
| Sex | F: 52 (42%) / M: 73 (58%) |
| Age at diagnosis (years): median, range | 52.0 (18.0-64.0) |
| Hb (g/dL): median, range | 12.5 (10.1-15.8) |
| Hb <12 g/dL | 40 (32%) |
| Plt (x109/L): median, range | 457.0 (47.0-1563.0) |
| Plt <150 x109/L | 13 (10%) |
| WBC (x109/L): median, range | 9.6 (2.2-23.5) |
| Spleen size (cm bcm): median, range | 4.0 (0.0-25.0) [1 NA] |
| Palpable | 93 (74%) |
| >5 cm bcm | 42 (34%) |
| Liver size (cm bcm): median, range | 1.0 (0.0-10.0) |
| Palpable | 64 (51%) |
| >2 cm bcm | 32 (26%) |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal