Abstract
BACKGROUND: There is high unmet need in frail pts with relapsed CLL, particularly in those characterized by adverse prognostic factors such as presence of del(17p) and/or TP53 mutations. Idelalisib is a first-in-class, targeted, highly selective, oral inhibitor of PI3Kd recently approved for the treatment of relapsed CLL in combination with R. This report describes results from the 2nd interim analysis (IA) of a Phase 3 study evaluating IDELA in combination with R in relapsed CLL with focus on various risk subgroups of pts.
METHODS: A Phase 3 study evaluated IDELA+R vs placebo (PBO)+R in pts with CLL requiring therapy after progression <24 mos since last therapy and considered unfit to receive cytotoxic therapy. Pts were randomized to receive IDELA at 150 BID (n=110) or PBO (n=110) in combination with R at 375 mg/m2 [1st dose] and then 500 mg/m2 q2 wks x 4, followed by q4 wks x 3 [8 doses total]). After progression, pts could enroll into a blinded extension study to receive IDELA at 150 mg BID (prior PBO+R) or 300 mg BID (prior IDELA+R). Primary endpoint PFS was assessed by an IRC using standard criteria (Hallek 2008/2012, Cheson 2012). The first IA (Furman, NEJM 2014) led to an early stop due to overwhelming efficacy. The 2nd IA was conducted at end of the blinded phase according to protocol (data cut-off Oct 9, 2013 with 63% of total PFS events) (Coutre, ASCO 2014). Samples for genetic analyses were analyzed by a central lab.
RESULTS: 220 pts (110/group) with median age of 71 yrs (78% ≥65 yrs), median time since diagnosis of 8.5 yrs, and median number of 3 prior therapies (range: 1-12) were randomized. 43% had del(17p)/TP53 mutation and 84% had unmutated IGHV.
At the time of data cut-off, the median (range) exposure was 5 (0-17) mos on IDELA+R and 4 (0-15) mos on PBO+R. 76% of pts on IDELA+R and 46% of pts on PBO+R were continuing on study.
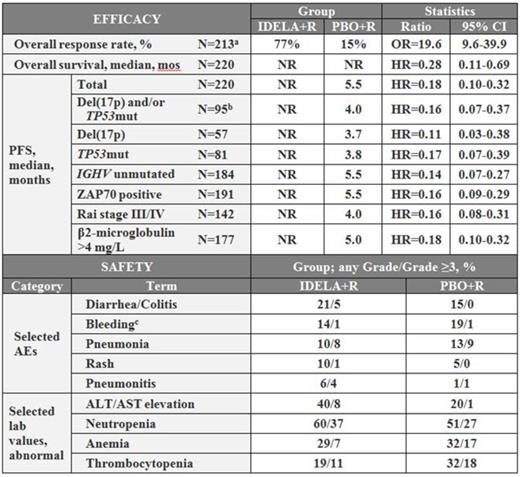
Median PFS was not reached for pts on IDELA+R and was 5.5 mos for pts on the comparator arm. At 12 months, the PFS rates for pts on IDELA treatment was 66% compared to 13% for pts on PBO+R. PFS strongly favored IDELA+R in all risk-subgroups, including those with genetic risk factors such as del(17p)/TP53 mutations, del(11q) (FIGURE 1) or unmutated IGHV as well as with disease-related risk factors like advance Rai stage or high levels of β2-microglobulin.
Adverse events (any Gr/Gr ≥3) occurring in ≥20% of pts on IDELA treatment were: pyrexia (IDELA+R 35%/3%; PBO+R 17%/1%), fatigue (IDELA+R 28%/3%; PBO+R 27%/2%), nausea (IDELA+R 26%/0%; PBO+R 21/0%), chills (IDELA+R 21%/2%; PBO+R 16%/0%), diarrhea/colitis (IDELA+R 21%/5%; PBO+R 15%/0%). ALT/AST elevation (any Gr/Gr ≥3) occurred in 40%/8% of pts on IDELA+R and in 20%/1% of pts on PBO+R. At the time of this analysis, 19 total deaths had occurred on the primary study: 6 on IDELA+R and 13 on PBO+R.
Summary of efficacy in various risk subgroups, and safety.
Summary of efficacy in various risk subgroups, and safety.
a) Number of patients on both arms evaluable at the time of analysis
b) Number of patients on both arms with respective test result. Note: Distribution of risk features on both arms was balanced.
c) Includes 16 preferred terms
KM estimates of PFS in pts with del(17p) or TP53mutation or del(11q) vs pts without any of these factors. IDELA+R without these risk factors: median PFS 12.1 mos (74% at 12 mos); IDELA+R with these risk factors: median PFS not reached (62% at 12 mos); PBO+R without these risk factors: median PFS 8.2 mos (19% at 12 mos); and PBO+R with these risk factors: median PFS 4 mos (8% at 12 mos). Curve comparison: Pts with these risk factors: HR = 0.16 (95% CI: 0.08, 0.32); Pts without these risk factors: HR = 0.24 (95% CI: 0.09, 0.66).
KM estimates of PFS in pts with del(17p) or TP53mutation or del(11q) vs pts without any of these factors. IDELA+R without these risk factors: median PFS 12.1 mos (74% at 12 mos); IDELA+R with these risk factors: median PFS not reached (62% at 12 mos); PBO+R without these risk factors: median PFS 8.2 mos (19% at 12 mos); and PBO+R with these risk factors: median PFS 4 mos (8% at 12 mos). Curve comparison: Pts with these risk factors: HR = 0.16 (95% CI: 0.08, 0.32); Pts without these risk factors: HR = 0.24 (95% CI: 0.09, 0.66).
CONCLUSIONS: IDELA+R demonstrated statistically significant improvement over PBO+R in PFS, ORR, and OS with an acceptable safety profile in these frail pts with relapsed CLL. These results confirm the 1st interim analysis of comparable efficacy of IDELA+R across all risk groups, including those with high-risk genomic markers like del(17p), TP53 mutations, and del(11q).
Sharman:Gilead Sciences: Research Funding. Coutre:Gilead Sciences: Research Funding. Furman:Gilead Sciences: Research Funding. Cheson:Gilead Sciences: Research Funding. Pagel:Gilead Sciences: Research Funding. Hillmen:Gilead Sciences: Research Funding. Barrientos:Gilead Sciences: Research Funding. Zelenetz:Gilead Sciences: Research Funding. Kipps:Gilead Sciences: Research Funding. Flinn:Gilead Sciences: Research Funding. Ghia:Gilead Sciences: Research Funding. Hallek:Gilead Sciences: Research Funding. Coiffier:Gilead Sciences: Research Funding. O'Brien:Gilead Sciences: Research Funding. Tausch:Gilead Sciences: Research Funding. Kreuzer:Gilead Sciences: Research Funding. Jiang:Gilead Sciences: Employment, Equity Ownership. Lazarov:Gilead Sciences: Employment, Equity Ownership. Li:Gilead Science: Employment, Equity Ownership. Jahn:Gilead Sciences: Employment, Equity Ownership. Stilgenbauer:Gilead Sciences: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal