Abstract
Introduction: Nurse-like cells (NLCs) are found in secondary lymphoid organs of Chronic Lymphocytic Leukemia (CLL) patients and represent an important component of the CLL microenvironment. NLCs can differentiate in vitro from monocytes after coculture of CLL cells with peripheral blood mononuclear cells and support the survival and proliferation of CLL cells through several mechanisms, including BCR signaling activation and CCL3/4 chemokine production.
Aim: The aim of the present study is to gain insight on the mechanism of BCR signaling activation in CLL-NLC coculture with a focus on the functional differences between IgM and IgD signaling.
Methods: BCR surface expression was analyzed by flow cytometry; CLL cells were stimulated with soluble anti-IgM or anti-IgD and 48-hour cell viability was evaluated by flow cytometry; CCL3/4 chemokine secretion was analyzed after 24-hour anti-IgM or anti-IgD stimulation by ELISA.
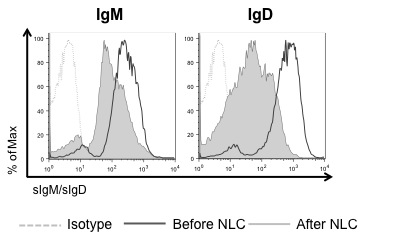
Results: We analyzed the levels of surface IgM (sIgM) and IgD (sIgD) expression on CLL cells before and after coculture with NLCs. Rapid downmodulation of both sIgM and sIgD was observed in CLL-NLC cocultures (Figure 1). CLL cells carrying unmutated IGHV genes (U-CLL) expressed significantly higher sIgM and sIgD than mutated CLL (M-CLL) (p(sIgM)<0.0001; p(sIgD)=0.0008; 35 samples/group) and downmodulated both receptors to a greater extent than M-CLL in the presence of NLCs (p(sIgM)=0.04; p(sIgD)=0.0047; 7 samples/group). Downmodulation of both sIgM and sIgD was not observed on CLL cells (6 patients) after coculture with the human mesenchymal stromal cell line hTERT, suggesting that receptor downregulation is a direct effect of the CLL-NLC interaction. Antigen stimulation is required for receptor internalization, as rapid downmodulation of both sIgM and sIgD was observed following BCR stimulation with soluble anti-IgM and anti-IgD in 14 samples.
Two outcomes of the CLL-NLC interaction are protection of CLL cells from apoptosis and CCL3/4 chemokine production. We replaced NLCs with anti-IgM and anti-IgD and examined the contribution of IgM and IgD signaling in mediating CLL cell survival and CCL3/4 secretion. IgM stimulation induced significantly higher protection from in vitro apoptosis as compared to IgD (p=0.03), as analyzed on 18 samples. In addition, U-CLL cells were more responsive to IgM-induced protection from apoptosis as compared to M-CLL (p=0.04, 9 samples/group). Both CCL3/4 were produced exclusively after anti-IgM stimulation, in particular of U-CLL; surprisingly, no CCL3/4 chemokine production was detected after anti-IgD stimulation, as analyzed on 12 samples. Simultaneous ligation of both IgM and IgD receptors did not affect viability and chemokine production, as compared to IgM stimulation alone.
Conclusion: Similar to what is observed when CLL cells are stimulated with soluble anti-IgM and anti-IgD, NLCs induce sIgM and sIgD downregulation on the surface of CLL cells, which is indicative of antigen engagement by the BCR. This effect appears to be NLC-specific, as it is not observed when CLL cells are co-cultured with the human mesenchymal stromal cell line hTERT. In vitro stimulation of CLL cells with anti-IgM induces greater protection from apoptosis and CCL3/4 chemokine production than anti-IgD stimulation, in particular in the U-CLL subset. Taken together, these results suggest that NLCs may present antigens to CLL cells, leading to IgM and IgD receptor engagement and downregulation. In this setting, IgM stimulation may be more relevant than IgD in the induction of NLC-specific protective effects, including CLL-cell survival and CCL3/4 production. Deeper insight into the nature of the CLL-NLC interaction and of the antigen(s) involved will provide a better understanding of NLC-mediated BCR triggering in CLL subsets, with therapeutic implications in the context of microenvironment or BCR signaling interference.
sIgM and sIgD expression levels on CLL cells before and after NLC coculture.
sIgM and sIgD expression levels on CLL cells before and after NLC coculture.
O'Brien:Amgen, Celgene, GSK: Consultancy; CLL Global Research Foundation: Membership on an entity's Board of Directors or advisory committees; Emergent, Genentech, Gilead, Infinity, Pharmacyclics, Spectrum: Consultancy, Research Funding; MorphoSys, Acerta, TG Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal