Abstract
BACKGROUND The Raf-1/MEK/MAPK-ERK1/2 pathway plays an important role in the pathogenesis and progression of multiple forms of cancer, including Chronic Lymphocytic Leukemia (CLL). Recent pre-clinical trials of the Raf-1 inhibitor Sorafenib demonstrate that targeting this pathway may represent a therapeutic option for CLL.
Raf-1 activity is negatively regulated by the Raf-1 kinase inhibitory protein (RKIP). However, in CLL cells evidence suggests that phosphorylation of RKIP by protein kinase C (PKC) may restrict RKIP binding to Raf-1 and promote its binding and inhibition of G-coupled protein receptor 2 (GRK2). Consequently, GRK2, which would normally bind AKT and MEK1/2, would no longer suppress activity of these proteins. It is conceivable, therefore, that deregulation of RKIP may contribute to the over activity of the Raf-1 and AKT-mediated pathways that has been described in freshly isolated CLL cells.
METHODS We sought to determine the effects of inhibiting RKIP on the survival and migratory capacity of CLL cells in vitro by using Locostatin, a specific inhibitor of RKIP binding to Raf-1 and GRK2. The proportion of apoptotic primary CLL cells following Locostatin treatment in either media alone or in the presence of a supportive stroma was assessed by flow cytometry. The mechanisms of action of Locostatin were investigated by western blotting for the phosphorylated forms of AKT and ERK1/2.
RESULTS Locostatin induced a dose dependent decrease in the proportion of viable cells from CLL patients (n = 6) and a panel of haematological lines (n = 8). The IC50 for Locostatin was significantly (P < 0.001) lower against primary CLL samples analysed (11.14 +/- 4.86 µM) than in peripheral blood mononuclear cells from healthy volunteers (72.71 +/- 9.85 µM). Locostatin was also effective against CLL cells cultured in the presence of a CD40L-expressing fibroblast layer.
Locostatin treatment reduced the expression of the chemokine receptor CXCR4 on the CLL cell surface with a concomitant decrease in their migration along a stroma-derived factor 1α (SDF-1α) gradient and significantly reduced the proportion of cycling CLL cells following induction by CpG oligonucleotide/IL2 (Dsp30/IL-2).
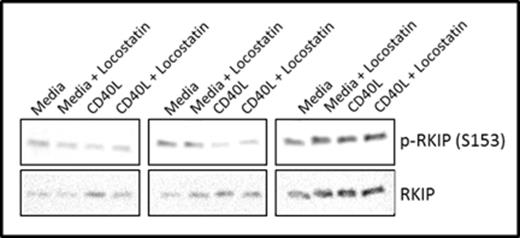
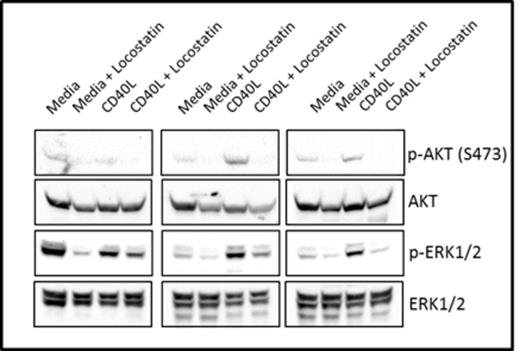
We demonstrate by western blotting that RKIP is phosphorylated on S153 in CLL patient samples (Figure 1), supporting our hypothesis that RKIP may not be able to bind Raf-1 in these cells. Furthermore, we show that Locostatin treatment inhibits phosphorylation of AKT (S473) and ERK1/2 in CLL cells cultured in media alone or on CD40L fibroblasts (Figure 2).
CONCLUSIONS These data suggest that deregulation of RKIP in CLL cells may contribute to their survival and proliferation by increasing the activities of AKT and ERK1/2. We show that Locostatin, a specific RKIP inhibitor, down-regulates the activity of both AKT and ERK1/2 in CLL cells cultured in media or a model of the tumor microenvironment, decreases cell viability and reduces the migratory and proliferative capacities of the leukemic cells. Our data suggest that RKIP may represent a novel target for therapy in CLL, which can be effectively inhibited by Locostatin.
Locostatin inhibits AKT and ERK1/2 phosphorylation in CLL cells cultured in media or on a CD40L-expressing fibroblast monolayer.
Locostatin inhibits AKT and ERK1/2 phosphorylation in CLL cells cultured in media or on a CD40L-expressing fibroblast monolayer.
Mulligan:Roche: Consultancy, Honoraria, Research Funding, Speakers Bureau; Sanofi Aventis: Research Funding; Janssen: Consultancy, Honoraria, Speakers Bureau; Celgene: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal