Abstract
Introduction: Experiments suggest that the anticoagulation effect of warfarin is mainly influenced by a reduction in coagulation factor (F) II and X activity and that FVII may have relatively little effect. Due to the short half-life of FVII but its strong influence on the prothrombin time (PT), monitoring warfarin using PT may therefore confound warfarin dosing. The Fiix-prothrombin time is specially designed to circumvent measuring FVII activity and is only sensitive to the activity of FII and FX in the sample. The Fiix-trial was a single center randomized prospective and blinded non-inferiority clinical trial that examined monitoring warfarin with the Fiix-PT compared to the PT.
Methods: All community dwelling patients 18 years and older with an INR target of 2-3 managed by our center´s anticoagulation management service were eligible for participation. A PT ratio adapted for thromboplastin sensitivity (INR) was calculated for both tests and results were reported by the laboratory as R-INR (“research INR”) to the dosing staff that was blinded to each patient´s test method. Warfarin dosing was software assisted using the DAWN anticoagulation software. Protocols designed for monitoring with PT were used, including a recommended maximum six week interval between tests. The major efficacy endpoint was fatal and non-fatal thromboembolism (TE) rate and the major safety endpoint was major bleeding per patient year (ppy). Surrogate efficacy parameters included number of tests, tests in range, time within target range 2-3 (TTR), monitoring test interval and dose adjustment rate.
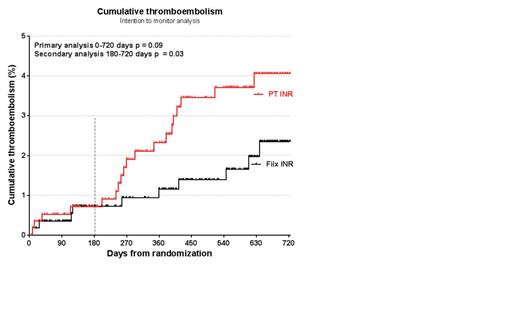
Results: After exclusion of 4 patients in each group, 573 patients were evaluable in the Fiix-PT arm and 575 in the control arm. About 69% had atrial fibrillation (AF) and 21% had venous thromboembolism (TE). The median monitoring time was 1.7 (IQR 1.1-1.9) years per patient in both study arms. The TE rate was similar in the first six months but thereafter the event curves diverged (Figure 1) and therefore we specially examined events occuring after six months in a secondary analysis. In the primary analysis of events during days 1-720, the TE rate ppy was 1.2% in the Fiix-arm vs 2.3% in controls (-48%, p = 0.09) demonstrating at least non-inferiority of Fiix-PT monitoring compared to the PT in reducing TE. The major bleeding rate did not differ; 2.2% and 2.5%, respectively (p = n.s., non-inferior). In the secondary analysis, after excluding the first six observation months, the Fiix-PT lead to a superior long-term reduction in TE (1.1% vs 2.2% annual rate, -50%, p = 0.03) with major bleeding rate of 1.5% vs 2.3% respectively (p = 0.8). A subanalysis of patients with AF yielded similar results. The intracranial hemorrhage (ICH)/intracerebral hemorrhage rate was 0.26%/0.13% ppy in the Fiix-arm and 0.64%/0.38% ppy in the PT-arm. In the first six months the test volume was the same in both arms but therafter a 5.8% reduction occurred in the Fiix-arm. The dose adjustment rate was reduced 12% overall and 18% during long term monitoring (4.1 vs 5.0 ppy, p = 0.01). At six month intervals, the median percent TTR was 84.9 (79.9-86.7) in the Fiix-arm vs 80.3 (78.5-81.3%) in the PT-arm.
Conclusion: Monitoring warfarin with Fiix-PT is non-inferior and possibly superior to the PT in reducing long-term recurrent TE. Bleeding is not increased despite omitting FVII activity in the monitoring test. With the Fiix-PT, the warfarin effect fluctuates less than with standard PT as manifested by a higher TTR and reduced dose-adjustment need. Overall, the results suggest that the fluctuation typically observed during warfarin treatment is partly caused by the traditional prothrombin time itself.
Onundarson:Fiix Diagnostics Ltd: Equity Ownership, I am a co-inventor of the Fiix prothrombin time and have stocks in Fiix Diagnostics, a startup company with the two inventors of the test as majority shareholders. The company is responsible for patent applications in process. Patents & Royalties. Gudmundsdottir:Fiix Diagnostics Ltd: Equity Ownership, I am a co-inventor of the Fiix prothrombin time and have stocks in Fiix Diagnostics, a startup company with the two inventors of the test as majority shareholders. The company is responsible for patent applications in process. Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal