Abstract
The BET inhibitors are first-in-class, epigenetic targeted therapies that deliver a new therapeutic paradigm by directly targeting protein-protein interactions at chromatin. Early clinical trials have shown significant promise, especially in AML, suggesting that these compounds are likely to form an important component of future anti-cancer regimens. Therapeutic resistance is an inevitable consequence of most cancer therapies, therefore the evaluation of resistance mechanisms is of utmost importance in order to optimize the clinical utility of this novel class of drugs.
Using primary murine stem and progenitor cells immortalized with MLL-AF9, we have developed a novel approach to generate over 20 clones stably resistant to the prototypical BET inhibitor, IBET. Resistance has been established at >IC90 of the parental cell line. In parallel, we have maintained matched vehicle treated clones in addition to the parental cell line. Resistant clones maintain their clonogenic capacity in IBET and are also impervious to IBET induced cell-cycle arrest and apoptosis. Resistance to IBET confers cross-resistance to other chemically distinct BET inhibitors such as JQ1 and also resistance to genetic knockdown of BET proteins. Moreover, resistance is stably maintained across subsequent cell generations in the absence of ongoing selective pressure.
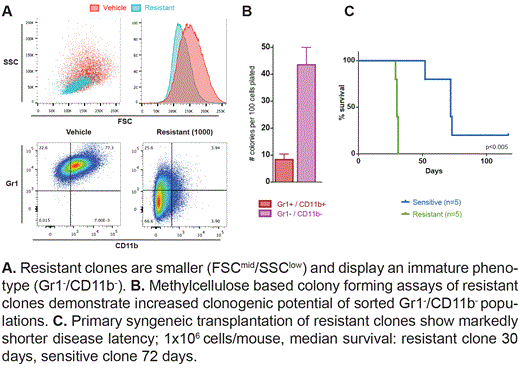
Resistance is not mediated through increased drug efflux or metabolism but is demonstrated to emerge from the leukemia stem cell (LSC) compartment. Resistant clones display an immature phenotype (c-kithi/Gr1-/CD11b-) and functionally, exhibit increased clonogenic capacity in vitro and markedly shorter disease latency following primary syngeneic transplantation (Figure A, B and C). Importantly, resistant clones maintain their resistance to IBET therapy in vivo.
We will present data gleaned from exome capture sequencing, ChIP-seq and RNA-seq, to demonstrate the underlying molecular mechanisms of resistance to epigenetic therapies, including genetic changes, molecular events at chromatin and the upregulation of compensatory pathways that will inform future combination therapies to obviate and/or overcome BET inhibitor resistance.
In summary, we have utilized a primary murine model of MLL leukemia to derive over 20 individual clones that are resistant to BET inhibition. Our data is consistent with resistance emerging from the LSC population. This data will allow us to develop rational drug combinations to overcome resistance and enhance the therapeutic efficacy of emerging epigenetic therapies. Furthermore, our data provides novel insights into the biology of AML and provides an unprecedented opportunity to study leukemia stem cells and develop therapeutic strategies to eradicate them.
Lugo:GlaxoSmithKline: Employment. Jeffrey:GlaxoSmithKline: Employment. Gregory:GlaxoSmithKline: Employment. Prinjha:GlaxoSmithKline: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal