Abstract
Introduction
Children with acute lymphoblastic leukemia (ALL) are at high risk for VTE due to several thrombotic risk factors such as the disease itself, central venous line (CVL), immobilization, infections and treatment with asparaginase and steroids, leading to increased morbidity and mortality. Identifying the clinical risk factors and high risk treatment phases for VTE is important and can lead to a better outcome and quality of life for these children. We conducted this prospective study on symptomatic VTE in children with ALL to characterize the prevalence, the clinical characteristics, and potential clinical predictive factors for symptomatic VTE and the impact of thrombosis on treatment delays.
Methods
All patients (n=1083), age 1-18 years, diagnosed with B-cell precursor or T-cell ALL between June 2008 and July 2013 and enrolled in the NOPHO ALL 2008 treatment protocol in the Nordic countries (Denmark, Finland, Iceland, Norway and Sweden), Estonia or Lithuania were included in the study.
Thrombotic events (TE) were prospectively recorded until the end of December 2013. TE was defined as objectively confirmed symptomatic TE. Main questions were: time of VTE occurrence, impact on treatment delay, type of CVL, dysfunction of the CVL, blood samples including D-dimers and thrombophilia screening, family history of TE, type and duration of antithrombotic therapy, and major bleeding during anticoagulation.
Results
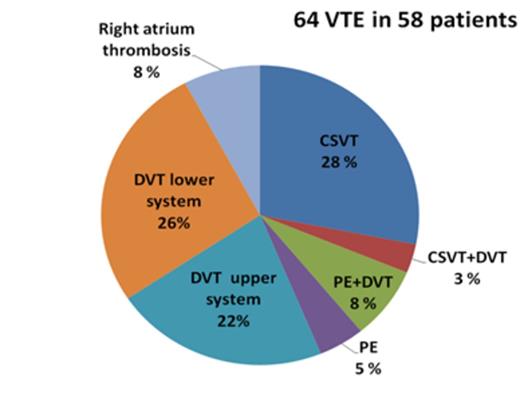
The cumulative risk of symptomatic VTE was 6.0% (CI 95% 4.7-7.7) for children treated with the NOPHO- ALL 2008 protocol. No arterial TE was found. VTE occurred in median 80 (IQR 43-118) days in the SR and 104 (IQR 39-127) days in the IR protocol and were in majority of cases associated with asparaginase treatment (84.5%, 49/58). See figure 1 for the localization of the VTE. VTE had a high impact on the treatment in the patients. Treatment with asparaginase was shortened in half of patients with VTE and chemotherapy treatment delayed in 25%. Age ≥ 15 years and residual disease ≥ 5% after induction therapy was significantly associated with VTE in the multivariate analysis (Table 1).
Conclusions
Our findings indicate that Venous Thromboembolism (VTE):
- is a major complication of ALL treatment
- may lead to reduced intensity of the ALL treatment and
subsequently possible long term impact on the EFS
- risk is dependent on the patients age and residual disease after leukemia induction
The possibility of identifying patients with elevated risk of VTE needs to be studied further and thromboprophylaxis for such patients during high-risk treatment phases can be considered in future ALL protocols.
Multivariate Cox regression analysis of the risk for VTE
| Factor . | HR (95% CI) . | P value . |
|---|---|---|
| Age category, years 1-7 8-14 15-17 | Ref 1.9 (1.0-3.7) 6.2 (3.4-11.3) | <0.000 0.044 0.000 |
| Gender Male | 1.6 (0.9-2.8) | 0.074 |
| ALL phenotype B-precursor T-cell Bilineage | Ref 2.3 (1.2-4.2) 4.2 (1.0-17.4) | 0.019 0.010 0.047 |
| Residual disease ≥ 5% day 29 | 4.1 (1.9-9.0) | 0.001 |
| Factor . | HR (95% CI) . | P value . |
|---|---|---|
| Age category, years 1-7 8-14 15-17 | Ref 1.9 (1.0-3.7) 6.2 (3.4-11.3) | <0.000 0.044 0.000 |
| Gender Male | 1.6 (0.9-2.8) | 0.074 |
| ALL phenotype B-precursor T-cell Bilineage | Ref 2.3 (1.2-4.2) 4.2 (1.0-17.4) | 0.019 0.010 0.047 |
| Residual disease ≥ 5% day 29 | 4.1 (1.9-9.0) | 0.001 |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal