Abstract
Background
Recent phase III data indicate that azacitidine (AZA) is active, and well tolerated, in patients with acute myeloid leukemia (AML) and baseline white blood cell (WBC) counts of <15G/L.1 However, few studies have assessed whether proliferative disease (WBC ≥15G/L) is a negative prognostic indicator in patients with World Health Organization (WHO)-AML treated with AZA.2,3
Methods
In this retrospective study of the Austrian AZA Registry (N=346), we assessed outcomes in patients with WHO-AML who received ≥1 dose of AZA, according to baseline WBC count. Patients were divided according to WBC <15G/L (n=297) and ≥15G/L (n=49), and AZA treatment line. Baseline characteristics and outcomes were compared.
Results
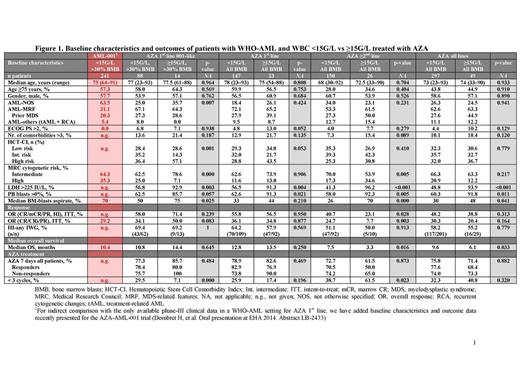
A comparison of baseline characteristics between the two groups revealed significantly higher levels of serum lactate dehydrogenase (LDH), peripheral blood (PB) and bone marrow (BM) blasts in patients with WBC ≥15G/L vs those with WBC <15G/L, indicative of more proliferative disease (Figure 1). Other baseline characteristics were evenly matched between the groups. Overall, both groups received a median of 4 AZA cycles (range: 1‒24 and 1‒46 in the WBC ≥15G/L and <15G/L groups, respectively). Similar patient numbers received AZA 1st line (<15G/L, n=147; ≥15G/L, n=23) or ≥2nd line (<15G/L, n=150; ≥15G/L, n=26).
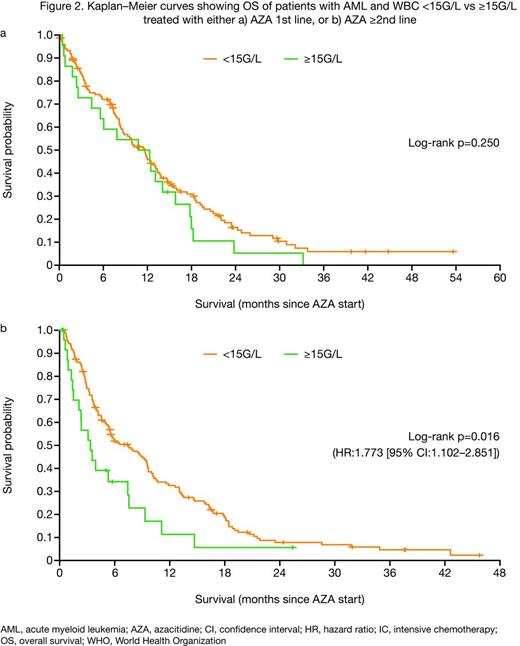
In the 1st line setting, overall response rate (ORR) was similar in the WBC <15G/L and ≥15G/L groups according to International Working Group (IWG) 20034 (36.1 vs 34.8%; p=0.877), IWG 20065 (36.1 vs 34.8%; p=0.569) or combined (complete response [CR]/CR with incomplete blood count recovery [CRi]/partial response [PR]/hematologic improvement [HI]) criteria (55.8 vs 56.5%; p=0.950; Figure 1). Median overall survival (OS) was 12.8 vs 13.5 months (p=0.250; Figure 2a), respectively.
In contrast, in the ≥2nd line setting, ORR was significantly higher in the WBC <15G/L than the ≥15G/L group (24.7 vs 7.7% [CR/CRi/PR], p=0.003; 40.7 vs 23.1% [CR/CRi/PR/HI], p=0.028). Median OS was higher in the WBC <15G/L group (7.5 vs 3.3 months; p=0.016; Figure 2b). Irrespective of treatment line, median response duration and relapse-free survival were numerically lower in the WBC ≥15G/L group.
Different baseline factors appeared to significantly impact OS in the </≥15G/L cohorts. In the <15G/L group, but not the ≥15G/L group, PB blasts >0% and LDH >225IU/L negatively affected OS, irrespective of treatment line. Adverse cytogenetics reached statistical significance for the whole <15G/L cohort and in the AZA ≥2nd line setting, whereas Eastern Cooperative Oncology Group Performance Status (ECOG PS) was significant for the whole <15G/L cohort and in the AZA 1st line setting. The only baseline factor that significantly impacted OS in the ≥15G/L cohort was number of comorbidities >3, primarily in the AZA 1st line setting.
Conclusions
Previous retrospective studies have suggested that WBC ≥15G/L has a negative impact on OS in AML patients treated with AZA.2,3 This report is the first to describe the impact of WBC (<15G/L vs ≥15G/L) on outcomes in patients who received AZA in both 1st or ≥2nd line settings and indicates that AZA can elicit favorable clinical outcomes, even in patients with elevated WBC counts when treated with AZA 1st line. In particular, a median OS of 13.5 months in patients with WBC ≥15G/L treated with 1st line AZA, despite elevated PB and BM blasts, as well as elevated LDH levels, is encouraging. These data suggest that AZA could be an effective treatment option in patients with proliferative AML in the 1st line setting. This is currently an unmet medical need; elderly patients with proliferative AML ineligible for intensive chemotherapy generally only receive best supportive care with hydroxyurea, and outcomes are poor. Based on these ‘real world’ data, randomized trials should assess AZA in patients with proliferative AML.
1. Dombret H, et al. Oral presentation at EHA 2014. Abstract LB-2433
2. Thepot S, et al. Am J Hematol 2014;89:410–6
3. van der Helm LH, et al. Leuk Res 2013;37:877–82
4. Cheson BD, et al. J Clin Oncol 2003;21:4642–9
5. Cheson BD, et al. Blood 2006;108:419–25
Pleyer:Celgene: Consultancy, Honoraria; AOP Orphan Pharmaceuticals: Honoraria; Novartis: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria. Off Label Use: Vidaza (azacitidine) is indicated for the treatment of adult AML patients who are not eligible for haematopoietic stem cell transplantation with 20–30 % blasts and multi-lineage dysplasia, according to WHO classification. This cohort also includes AML-patients with >30% bone marrow blasts.. Burgstaller:AOP Orphan Pharmaceuticals: Honoraria; Novartis: Honoraria; Mundipharma: Honoraria; Celgene: Consultancy. Stauder:Ratiopharm: Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Novartis: Research Funding. Girschikofsky:Pfizer: Honoraria, Research Funding; Mundipharm: Consultancy, Honoraria. Pfeilstöcker:Janssen-Cilag: Honoraria; Novartis: Consultancy, Honoraria; Celgene: Consultancy, Honoraria. Lang:Celgene: Consultancy. Sperr:Phadia: Research Funding; Novartis: Honoraria; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Valent:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Greil:Amgen: Honoraria, Research Funding; Eisai: Honoraria; Mundipharma: Honoraria, Research Funding; Merck: Honoraria; Janssen-Cilag: Honoraria; Genentech: Honoraria, Research Funding; Novartis: Honoraria; Astra-Zeneca: Honoraria; Boehringer-Ingelheim: Honoraria; Pfizer: Honoraria, Research Funding; Roche: Honoraria; Sanofi Aventis: Honoraria; GSK: Research Funding; Ratiopharm: Research Funding; Celgene: Consultancy, Research Funding; Cephalon: Consultancy, Honoraria, Research Funding; Bristol-Myers-Squibb: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal