Abstract
Introduction: While clinical studies using targeted therapies as single agents in AML have shown promising results in recent years, long-term durable responses in this aggressive cancer may require combination therapies to overcome disease progression and single agent resistance mechanisms. PLX3397 is an orally active, selective small molecule inhibitor of the constitutively activated FLT3-ITD mutant kinase. In cellular assays PLX3397 effectively inhibited FLT3-ITD autophosphorylation and FLT3-ITD driven proliferation with IC50s in the 10-100nM range. A clinical study to evaluate the pharmacokinetics (PK), safety and efficacy of PLX3397 in patients with FLT3-ITD AML is currently ongoing. In order to determine if combination therapy could improve efficacy, we evaluated the combination of PLX3397 with the hypomethylating agent decitabine (DEC; 5-aza-2’-deoxycytidine) in preclinical models of FLT-ITD AML. Decitabine, a drug originally indicated for myelodysplastic syndrome, is approved in Europe for the treatment of adult patients (≥65 years of age) with newly diagnosed or secondary AML.
Methods: For the in vitro growth assays, cells were pre-treated with decitabine for 0-3 days prior to the addition of PLX3397. Following a 3-day incubation, cell viability was measured based on quantification of the ATP present. The resulting data were analyzed for synergy and combination indices were calculated using CalcuSyn software. Apoptosis was analyzed by measuring caspase 3/7 activity following a 24h incubation with both compounds. For the in vivo study, MV-4-11 cells were grown as subcutaneously implanted xenografts in SCID mice. When tumors reached a size of ~500 mm3 the mice were randomized into equal-sized treatment groups by body weight and tumor size (the day on which this was done was counted as day 0). Decitabine was dosed at 20mg/kg on days 1, 7, 13 and 20 after randomization. PLX3397 was dosed at 20mg/kg on day 2, and continued for 20 days. The combination followed the same dosing schemes as the two single agents.
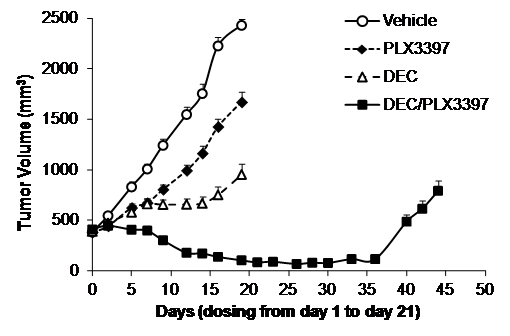
Results: In vitro viability experiments in two AML cell lines (MV-4-11 and MOLM14) using a dose matrix format demonstrated a combination benefit of PLX3397 and decitabine over a range of concentrations. Pre-incubation with decitabine for 3 days prior to the addition of PLX3397 enhanced the synergy observed. PLX3397 alone was more effective than decitabine at inducing apoptosis. Adding both compounds together slightly enhanced the induction of apoptosis, though there did not appear to be an added benefit to pre-treating the cells with decitabine, as was seen in the viability assays. To confirm the synergy observed in vitro we tested the in vivo efficacy of the two agents in the MV-4-11 xenograft model. By day 19, both decitabine and PLX3397 delayed tumor growth, resulting in tumor growth inhibition (TGI) of 89% and 42%, respectively. The combination of decitabine and PLX3397 showed striking antitumor activity, causing tumor regression and reducing tumor volume by 88%. This tumor suppression was maintained for 15 days after the treatment was stopped. Consistent with clinical experience, decitabine treatment was associated with bone marrow toxicity. This toxicity was not worsened by PLX3397. After 2 weeks of recovery bone marrow cellularity rebounded to pre-dosing levels in the combination, with the exception of red blood cell count.
Conclusion: Preclinical studies of PLX3397 and decitabine in FLT3-ITD AML cell lines and a xenograft model demonstrated beneficial effects when used in combination. Single agent treatment inhibited MV-4-11 xenograft tumor growth, while the combination resulted in tumor regression. PLX3397 did not further enhance the bone marrow toxicity induced by decitabine. PLX3397 exposures in these preclinical studies are similar to those achieved in AML patients in the on-going single agent clinical trial.
Preclinical combination of PLX3397 and decitabine in an MV-4-11 xenograft model.
Preclinical combination of PLX3397 and decitabine in an MV-4-11 xenograft model.
Zhang:Plexxikon: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal