Abstract
Background:
Imbalances in iron homeostasis result in a variety of disorders. Excess iron accumulates in the circulation and tissues of patients with hereditary hemochromatosis (HH), iron-loading anemia (β-thalassemia major and intermedia (Thal), myelodysplastic syndromes (MDS) and sickle cell disease after transfusion (SCD). To prevent iron-induced tissue damage, detection of impending iron toxicity is needed before complications develop and become irreversible. Plasma non-transferrin bound iron (NTBI) and its labile (redox active) component (LPI) are thought to be potentially toxic forms of iron identified in the serum of patients with iron overload.
Objective:
To increase our insights into NTBI and LPI concentrations measured by the current worldwide leading analytical assays in four different categories of iron overloaded patients (HH, Thal, MDS, transfused SCD) undergoing various treatments (phlebotomies, iron chelation, red blood cell transfusions).
Methods:
We compared 10 different assays (5 NTBI, 1 NTBI isoform specific and 4 LPI) as part of an international inter-laboratory study. Serum samples were from 60 patients with 4 iron overload disorders. Serum samples were split into two aliquots, coded (blinded), stored at -80°C and shipped for analysis to 5 different laboratories worldwide. Laboratories performed duplicate measurements on each aliquot of a serum sample on 2 different days, resulting in a total of 4 measurements for each sample. Some laboratories provided multiple assays.
Results:
NTBI and LPI measurements in the serum of iron-overloaded patients showed good reproducibility with a high between-sample (range 67.1-97.2%) and a low within-sample variance (0-2.2%) relative to the total variance of each assay. Absolute NTBI and LPI levels differed considerably between assays. Four assays (2 LPI and 2 NTBI) also reported negative values. LPI levels were ± 10% of the NTBI levels. Highest levels were observed in patients with naive HH and naive Thal intermedia, transfusion-dependent MDS and transfusion-dependent Thal major. These 4 patients groups also had the highest transferrin saturation (TSAT) levels, but only 3 of them were among the groups with the highest ferritin levels. Eight (4 LPI and 4 NTBI) of the 10 assays could discriminate well between iron overload diseases.
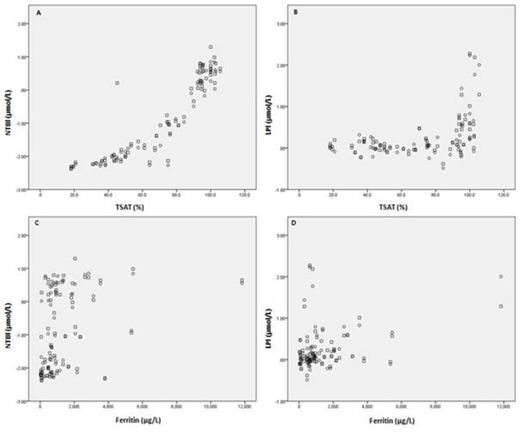
In general correlations were highest within the same group of NTBI or LPI assays. Interestingly, one of the LPI assays showed better correlations with NTBI assays (range rs=0.85-0.90) than with the other LPI assays (range rs=0.61-0.77). In contrast, one of the NTBI assays showed better correlations with LPI assays (range rs=0.67-0.75) than with the other NTBI assays (range rs=0.50-0.59). The assays show a hyperbolic relation with TSAT; NTBI and LPI concentrations only substantially increase above a certain TSAT level of ~70% and ~ 90%, respectively. This is illustrated for both a representative NTBI (Figure 1A) and LPI assay (Figure 1B). This relation does not exist between any of the assays and ferritin (Figure 1C,D).
Conclusions:
While NTBI and LPI values of various assays are well correlated and discriminate between iron overload disorders, absolute values differed considerably between assays. Both standardization of assays and clinical outcome studies to determine clinically relevant toxic thresholds are needed. At present TSAT may provide a useful alternative in the clinical management of patients with iron overload.
Relation between representative assays and TSAT, Ferritin. Assay results are given for day 2 as duplicate measurements (circle and square).
Relation between representative assays and TSAT, Ferritin. Assay results are given for day 2 as duplicate measurements (circle and square).
De Swart:Novartis Europe: Research Funding. Swinkels:Novartis Europe: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal