Abstract
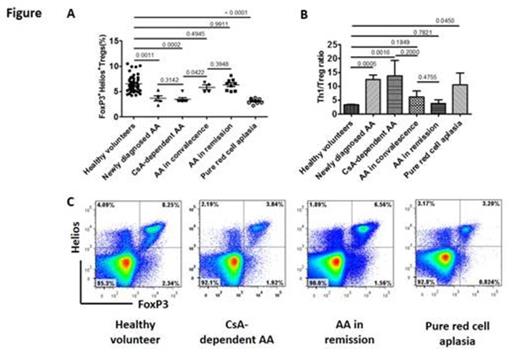
Background: A subset of patients with acquired aplastic anemia (AA) require low-dose cyclosporine (CsA) to maintain good hematopoietic function even after achieving a remission with immunosuppressive therapy (IST). Such CsA-dependent AA offers opportunities to clarify the immune mechanisms underlying bone marrow (BM) failure, but little is known about the mechanism(s) underlying this condition except for its close association with a particular HLA-class II allele DRB1*15:01. Regulatory T cells (Tregs), defined as CD4+CD25highFoxP3+ T cells, have been shown to be decreased in patients with severe AA at diagnosis and to recover to normal levels in association with hematological recovery after successful IST. An insufficient Treg recovery may account for dependency on CsA in a particular subset of AA patients, and also in patients with idiopathic pure red cell aplasia (PRCA), another typical immune-mediated BM failure of which remission is known to depend on CsA in virtually all patients. To test this hypothesis, we determined the percentage and absolute number of Tregs, as well as Th1, Th2 and Th17 cells, in various types of immune-mediated BM failure, using an anti-FoxP3 antibody (Ab) and anti-Helios Ab that allows enumeration of the functional Tregs more precisely than conventional methods. Methods: Different CD4+ Treg subsets were enumerated using a multicolor flow cytometry assay. Peripheral blood mononuclear cells (PBMCs) were stimulated by phorbol myristate acetate (PMA) and ionomycin in the presence of monensin for four hours, then were subjected to flow cytometry to determine the percentage of CD4+ T cells positive for cytoplasmic interferon-γ (Th1), interleukin 4 (Th2) and interleukin 17 (Th17). CsA-dependent AA was defined as AA with two or more episodes of relapse of pancytopenia that occurred during more than one year of therapy, followed by a good response to CsA. All six patients with CsA-dependent AA had normal blood cell counts and HLA-DR15 (DRB1*15:01 in five and DRB1*15:02 in one). Results: The normal ranges of each T cell subset percentage and the absolute numbers determined in 47 healthy volunteers (HVs) were: FoxP3+Helios+ Tregs 6.5±1.6%/ 47.7±13.8/µl, Th1 22.3±6.6%/ 151.2± 45.5/µl, Th2 5.5±1.6%/ 38.3±170.9/µl and Th17 1.7± 0.7%/ 11.2± 4.7/µl. The expression of FoxP3 by CD4+ T cells decreased with time after sampling, and this assay therefore necessitated analyses within 16 hours after sampling using fresh PBMCs. Compared to healthy volunteers, the percentage and absolute number of FoxP3+Helios+ Treg cells were significantly lower in the five patients with severe AA at diagnosis (3.7±1.1%, 22.7±6.8/µl) and in six CsA-dependent AA patients (3.4±0.8%, 13.8±12.3/µl) (Figure). In contrast, the Treg percentages were comparable to those of the HVs in four AA patients on CsA in convalescence (5.8±1.0%) and nine patients in remission off CsA (6.3±1.1%) while the absolute numbers of FoxP3+Helios+Tregs (27.3±19.2/µl, 32.3±13.3/µl) in these groups were lower than those of the HVs. On the other hand, the percentages of all other CD4+ T cell subsets were comparable among the four different groups and HVs. The apparent decrease in FoxP3+Helios+ Tregs despite normal blood cell counts in patients with CsA-dependent AA prompted us to screen nine patients with idiopathic PRCA whose erythropoietic functions were all dependent on CsA. The FoxP3+Helios+ Tregs were decreased in both the percentage and absolute count (3.1±0.5%, 12.1±5.9/µl) in all of these patients, including two patients whose PBMCs were examined before CsA therapy. As a result, the ratios of Th1/Treg and Th17/Treg were markedly higher in newly-diagnosed AA (12.5±3.4, 0.9±0.8), CsA-dependent AA (12.6±12.1, 1.1±1.5) and PRCA (10.6±7.7, 0.7±0.3) patients than in AA patients in convalescence (6.1±4.3, 0.4±0.1) and those in remission (3.8±2.3, 0.4±0.2), which were comparable to the HVs Th1/Treg and Th17/Treg ratios (3.3±0.9, 0.3±0.2). Conclusions: Decreased percentages and absolute counts of Tregs, as well as increased Th1/Treg and Th17/Treg ratios, are common features in patients with CsA-dependent AA and PRCA. A persistent decrease in Tregs may serve as a good marker for predicting AA relapse in patients responding to CsA.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal