Abstract
Background: PML is an often fatal demyelinating disease of the brain due to reactivation of latent JC polyoma virus. Rituximab is an anti-CD20 monoclonal antibody associated with PML, as outlined briefly in a revised Black Box warning in 2007 and a series of Dear Healthcare Professional Letters sent by the manufacturer between 2006 and 2008. In 2009, we described 57 HIV-negative patients who developed PML after rituximab treatment [Blood. 2009; 113(20):4834–40]. Since then, the use of rituximab has continued to increase as has awareness of this association.
Methods: A Freedom of Information (FOI) Act request was submitted to the U.S. Food and Drug Administration (FDA) for reports of PML cases occurring in rituximab-treated patients. These reports were obtained from the FDA in September 2013, and by definition of the FOI request, were at least 6 months old at the time obtained. Of the 483 total reports received, 173 duplicate cases were identified based on similar dates, ages, and other clinical information, resulting in 310 unique cases. An additional 14 cases were added from our 2009 publication that were not included in the original 310 cases, totaling 324 unique cases. Our case definition included HIV-negative patients in whom rituximab was administered prior to the onset of PML symptoms, with PML confirmed via brain biopsy, autopsy, or having JC virus in cerebrospinal fluid (CSF) plus brain MRI findings of PML.
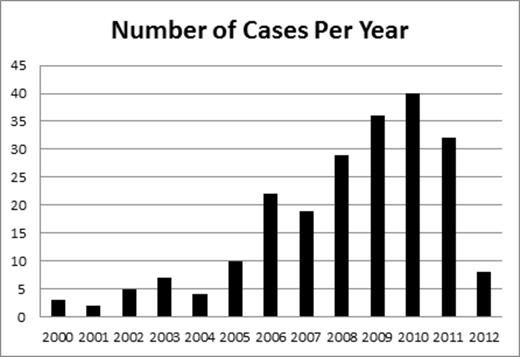
Results: Of the 324 unique cases, there were 231 confirmed cases of rituximab-associated PML in HIV-negative patients, from 23 countries. The majority of excluded cases lacked the necessary details in the reports to confirm the diagnosis of PML. Among the confirmed cases, case mortality rate was 89.1%. PML diagnosis was made by brain MRI AND positive JC virus CSF PCR (67.5%), brain biopsy (34.6%), or autopsy (7.8%). Indications for rituximab use included non-Hodgkin lymphoma (58.8%), chronic lymphocytic leukemia (28.4%), rheumatoid arthritis (3.1%), systemic lupus erythematosus (2.6%), autoimmune hemolytic anemia/idiopathic thrombocytopenic purpura (2.6%), and other rheumatologic conditions (3.5%). The non-Hodgkin lymphomas were subdivided into follicular (13.5%), diffuse large B-cell (9.2%), unspecified non-Hodgkin (22.7%), mantle cell (7%), Waldenstrom (4.4%), and marginal zone (1.7%). Median age was 64.5 years (range 19-90), 53% female and 47% male, with 25 patients (10.8%) having undergone prior transplantation (12 autologous stem cell, 8 allogeneic stem cell, and 5 solid organ). The median number of rituximab doses received was 6 (range 1-24), with a median of 15 months (range 0.5-128) from first dose of rituximab to PML diagnosis, and median of 4 months (range 0-65) from last rituximab dose to PML. The median time from PML diagnosis to death was 2 months (range 0-14). It is noteworthy that of the cases diagnosed by brain biopsy or autopsy, 17 had a negative JC virus CSF PCR, and of the cases diagnosed with a positive JC virus CSF PCR, 8 had at least one negative test before the diagnosis was made. Less than 10 cases per year were diagnosed until 2006 when there were 22, peaking at 40 in 2010 (figure).
Conclusions: PML continues to be increasingly reported by clinicians who administer rituximab with or without concomitant chemotherapy. The temporal increase in the number of reports suggests that either the incidence may be higher than initially thought or increased awareness has led to more reporting. Furthermore, the lower number of reports in 2012 likely illustrates the delay between incident cases, reporting, and availability through FOI requests. Our findings highlight the importance of detailed adverse event reporting (as is characteristic of SONAR) to enhance clinician awareness of rare but serious toxicities, as well as the need for faster public availability of adverse event reports.
Off Label Use: Rituximab is used for a variety of indications. Our abstract describes the indications with percentages that rituximab was used from our analysis. These FDA-approved indications include chronic lymphocytic leukemia, lymphomas as listed, and rheumatoid arthritis. Indications listed that are not FDA-approved (the minority) include systemic lupus erythematosus, autoimmune hemolytic anemia/idiopathic thrombocytopenic purpura, and other rheumatologic conditions.. Carson:Genentech: Consultancy, Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal