Abstract

Background: Chronic myeloid leukemia (CML) is characterized by the expression of the BCR-ABL fusion protein in virtually all malignant cells in the vast majority of patients. This molecular specificity forms the basis of a highly efficient, targeted therapy by TKIs. The success of this therapeutic approach is the reason that CML has developed into a showcase example for an efficient, targeted tumor therapy.
Methods: As previously shown, the application of a single-cell–based mathematical model, which describes CML as a clonal competition between normal and leukemic hematopoietic stem cells, can consistently describe the median long-term BCR-ABL transcript levels in response to the first-generation TKI imatinib [1]. Furthermore, it has been demonstrated that a similar modeling approach also allows predicting the molecular long-term response to imatinib in individual patients [2]. Specifically, we showed that these predictions, including the determination of an optimal, patient-specific time point for potential treatment cessation, can be derived directly from time course data of the BCR-ABL transcript levels. Here we present novel results on the application of a similar analysis strategy for CML patients, treated with the second-generation TKI dasatinib. Based on unpublished data describing the 4-year molecular response dynamics (ie, BCR-ABL transcript levels) observed in a randomized, controlled clinical trial (DASISION [3]), we directly compare the effects of dasatinib (n=253) and imatinib (n=255).
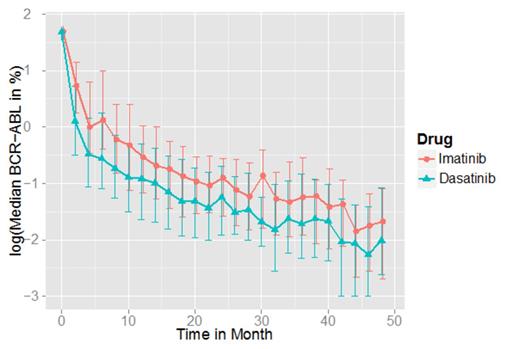
Results: These data show an accelerated and deeper median molecular response in the dasatinib arm (Figure 1). We confirmed this observation by statistical analysis, describing the BCR-ABL response kinetics by a bi-phasic exponential model. Comparing different characteristic parameters of this model, we could demonstrate a significantly steeper initial decline in the dasatinib arm compared to imatinib (P < 0.001, t-test on mean initial decline). Beyond the statistical analysis, we apply the above-mentioned single-cell–based mathematical model. Adapting its parameters to the specific kinetic characteristics observed under the different treatment conditions, model predictions for both the population and individual patients are derived for both treatment scenarios. In our presentation we will compare the model predictions of long-term BCR-ABL kinetics under first line imatinib and dasatinib treatment. The population and patient-specific model predictions will include BCR-ABL levels in the peripheral blood and bone marrow, as well as the estimated risk of molecular relapses after stopping treatment and, related to this, optimal time points for potential treatment cessation.
Conclusion: Our analysis suggests that the application of model-prediction tools, previously used for the first-generation TKI imatinib, can be applied to other TKIs. The steeper initial decline in the dasatinib arm may be relevant in estimating relapse risk, which will be determined from long-term outcome of ongoing discontinuation trials.
[1] Roeder I et al. (2006) Nat. Med.
[2] Horn M et al. (2013) Blood.
[3] Cortes JE et al. ASH 2013, Abstract #653.
Observed median molecular response dynamics of TKI-treated CML patients. Error bars represent inter-quartile ranges.
Observed median molecular response dynamics of TKI-treated CML patients. Error bars represent inter-quartile ranges.
Glauche:Bristol-Myers Squibb: Research Funding. Fröhlich:Bristol-Myers Squibb: Employment. Roy:Bristol-Myers Squibb: Employment, Equity Ownership. Subar:Bristol-Myers Squibb: Employment. Wang:Bristol-Myers Squibb: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal