Abstract
Introduction
DNA methyltransferase (DNMT) family play an important role in the development and growth of lives, encoding enzymes that catalyze the addition of a methyl group to the cytosine residue of CpG islands. With the increase in methylation, the downstream genes are often associated with reduced expression. In this family, DNMT3a occupies the essential position to implement the de novo methylation. Timothy J. Ley and many other scientists found that in M4 and M5 acute myeloid leukemia (AML), around 20% patients suffered from DNMT3a mutation (most are R882H mutation), always associating with adverse prognosis. But what's the reason for adverse prognosis? Additionally, our formal Meta analysis showed that the de novo AML patients with DNMT3a mutation have higher platelet counts, WBC and RBC counts. To shed some light on the possible causal relation between the increasing in platelet count and poor prognosis led by DNMT3a mutation, we transduced the MK cell lines with genes null-mCherry (null), DNMT3a wild type-mCherry (DNMT3aWT) and DNMT3a R882H mutation type-mCherry (DNMT3aMT) respectively, trying to figure out the possible role that the mutation plays in the megakaryopoiesis and thrombopoiesis. Also, we tested several drugs that may target the mutation.
Methods
The SFFV-null-IRES-mCherry, SFFV-DNMT3aWT-IRES-mCherry and SFFV-DNMT3aMT-IRES-mCherry plasmids were constructed by Dr. Qianli Jiang, modified from LEGO-iC plasmids. MK cell lines (chrf-288-11, meg-01) were flow-through transduced with the lentivirus produced by packaging plasmids and those above. All the fluorescence positive cells have been doubly sorted by flow cytometry. Cell ploidy was analyzed by flow cytometry using Propidium Iodide (PI); colony forming unit (CFU-MK) were enumerated 14d after being plated with TPO and IL-3; cell proliferation were tested by CCK-8; apoptosis was measured via flow cytometry with PI and Annexin V-FITC; CD41a and CD61 were tested with flow cytometry. The drug tests including Decitabine, Dasatinib and Rituximab were analyzed using CCK-8 test and cytomorphologic tests.
Results
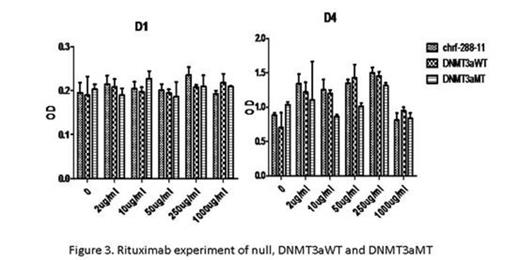
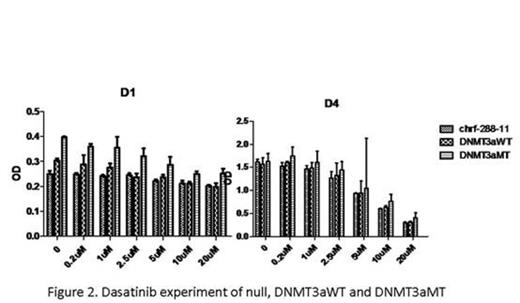
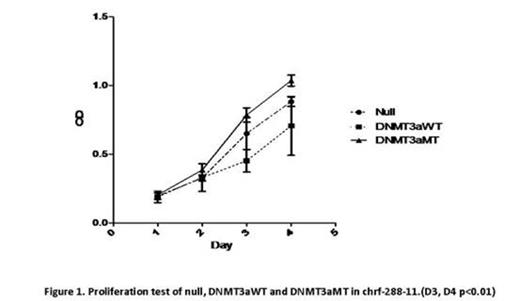
With CCK8 test of chrf-288-11 and meg-01, DNMT3aMT proliferates faster than the null and DNMT3aWT (P<0.05, Fig.1). In CFU-MK, both cells lines showed that DNMT3a mutation promoted the colony formation (P<0.05). The CD41a percentage decreased from null to DNMT3aWT and DNMT3aMT (P<0.05) while the CD61percentage increased from null to DNMT3aWT and DNMT3aMT (P<0.05). Also, with morphologic analyses, DNMT3aMT in both cell lines maintain more mature stages. Cell ploidy test also demonstrated that cell lines with DNMT3a mutation contain more multiploids (P<0.05). Apoptosis test illustrated that DNMT3a mutation protect the cell lines from apoptosis (P<0.05). In the drug experiments, 1uM Decitabine could slow down the proliferation of 3 gene types of chrf-288-11 significantly (P<0.05). Dasatinib also posed a negative effect on the proliferation of 3 gene types of chrf-288-11 (P<0.05, Fig.2). In Rituximab experiment, we could find that interestingly, certain concentrations could speed up the proliferation of 3 gene types of chrf-288-11, while others not (P<0.05, Fig.3).
Conclusion
With all the above evidences, we can safely conclude that the megakaryocyte cell lines with DNMT3a mutation are associated with high-differentiation, high-colony formation and low-apoptosis, which could help us to understand the elevation of platelet count in AML patients with DNMT3a mutation. The anti-apoptosis and renewal ability of the cell lines with DNMT3a mutation may lead to a bad prognosis of these AML patients (with DNMT3a mutation). What's more, according to the drug experiment, we found in the both cell lines, DNMT3aWT and DNMT3aMT cells died significantly at even low concentration of decitabine. Dasatinib also slowed down the proliferation of 3 gene types of chrf-288-11, whether Dasatinib could lead to further treatment of such leukemia with DNMT3A mutation needs more research. Rituximab is helpful in the treatment against refractory thrombocytopenia. However, the mechanism hasn't been clarified. Interestingly, our results showed that, Rituximab may have a direct effect on MKs, giving a boost to the megakaryopoiesis and thrombopoiesis with certain concentration.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal