Abstract
Deletion of the long arm of chromosome 20 [del(20q)] is a common recurrent chromosomal abnormality in acute myeloid leukaemia (AML) and myeloproliferative neoplasms (MPN). The abnormal chromosome 20 has a “Common Deleted Region” (CDR) that contains Protein Tyrosine Phosphatase Receptor T (PTPRT), a tyrosine phosphatase that is mutated in many human cancers. We have previously reported (MacKinnon et al, Genes, Chromosomes and Cancer 2010) that del(20q) also harbours an amplified “Common Retained Region,” (CRR) which contains multiple copies of Haemopoietic Cell Kinase (HCK). HCK is an oncogenic Src tyrosine kinase and its aberrant activation has been shown to contribute to the pathogenesis of some haematological malignancies.
Our hypothesis is that the amplification of HCK in the CRR cooperated with the loss of PTPRT in the CDR. Indeed, our results strongly suggested that HCK amplification with PTPRT loss led to a myeloproliferative phenotype seen in MPN.
Our model proposed that MPN occurred either through the consequence of direct interaction between HCK and PTPRT, or through aberrant activation of Signal Transducer and Activator of Transcription 3 (STAT3). Constitutively activated STAT3 has been shown to be oncogenic in several haematological malignancies. STAT3 is a direct target of both HCK and PTPRT. It is phosphorylated (hence activated) by HCK, and dephosphorylated (hence inactivated) by PTPRT. This provides a downstream leukaemogenic pathway for our model.The ultimate aim of our experiments is to prove this hypothesis using mouse models. Murine haemopoietic stem cells (HSC) were isolated from the bone marrows of wild type C57BL/6 (WT) and PTPRT-null mice by Fluorescence Activated Cell Sorting for Lineage negative, C-kit and Sca-1 positive (LKS+) cells. Retroviral constructs of HCK were generated by cloning it into the retroviral vector pMSCViresEGFP(MIG), with GFP as reporter. Murine HSC were transduced with either retroviral HCK or MIG vector control. Hence wild type (WT) and PTPRT-null murine HSC transduced with either MIG or HCK were used in our experiments (WTMIG, WTHCK, PTPRTMIG, PTPRTHCK).
We have previously presented that PTPRTHCK showed a significant increase in methylcelluose colony numbers and colony sizes compared to PTPRTMIG, while there was no difference between WTMIG and WTHCK. In addition, an intracellular anti-phosphoSTAT3 antibody assay demonstrated that in both WTHCK and PTPRTHCK, significant STAT3 hyperphosphorylation, and hence overactivation, occurred. This response was more exaggerated in the PTPRTHCK. Finally, direct interaction between HCK and PTPRT was shown with an interaction assay using recombinant proteins. We now present the in vivo data in the murine recipients.
In Vivo Reconstitution
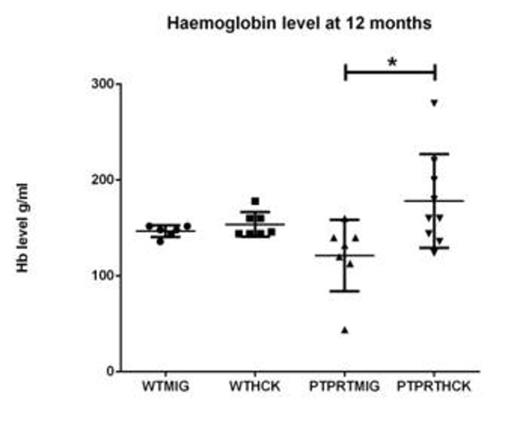
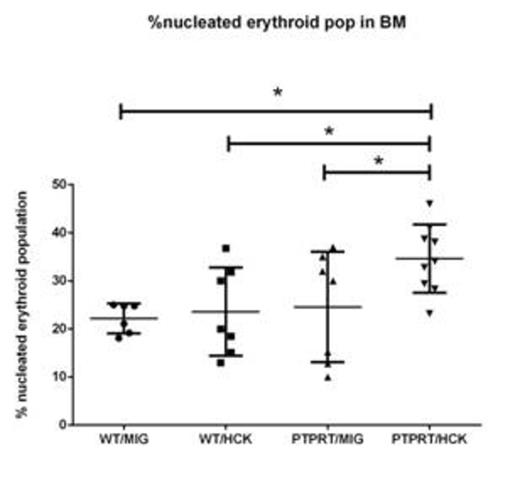
LKS+ HSC transduced with either MIG or HCK were transplanted into lethally irradiated SJL/PTPRCa murine recipients to assess AML or MPN formation in a reconstitution study over 12 months. The recipients from the four cohorts (WTMIG, WTHCK, PTPRTMIG and PTPRTHCK) were regularly analysed by full blood counts, peripheral blood GFP%, and blood films. At 12 months, they were culled and the organs harvested and analysed by flow cytometry, cytospin, and paraffin-embedded histology (H + E and immunohistochemistry). Recipents of PTPRTHCK HSC had a significantly higher proportion of nucleated erythroid populations in the bone marrow by flow and larger splenic size by weight. They also showed a higher haemoglobin (Hb) at 12 months, although this did not reach statistical significance.
Haemoglobin level of the recipient mice. PTPRTHCK recipients had a higher average Hb compared to the others, although only reaching statistical significance when compared to PTPRTMIG recipients.
Haemoglobin level of the recipient mice. PTPRTHCK recipients had a higher average Hb compared to the others, although only reaching statistical significance when compared to PTPRTMIG recipients.
%Nucleated erythroid population in bone marrow by flow. PTPRTHCK recipients had a higher % of nucleated erythroid cells compared to the others
%Nucleated erythroid population in bone marrow by flow. PTPRTHCK recipients had a higher % of nucleated erythroid cells compared to the others
Splenic size of recipient mice at 12 months. PTPRTHCK recipients had a larger splenic size compared to others, although this was just outside statistical significance when compared to WTHCK.
Splenic size of recipient mice at 12 months. PTPRTHCK recipients had a larger splenic size compared to others, although this was just outside statistical significance when compared to WTHCK.
Conclusion
The data reveal a likely new oncogenic-signalling cascade: that HCK amplification and loss of PTPRT in del(20q) may cooperate to cause MPN directly, or by aberrant activity of hyperphosphorylated STAT3.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal