Abstract
Background: With currently available therapies, relapsed/refractory aggressive non-Hodgkin lymphoma (NHL) after high-dose therapy or, in not transplant-eligible patients, after first-line chemotherapy represents an unequivocally unmet clinical need. Aim and Methods: Therefore, we aimed at evaluating a combination chemotherapy regimen based on pixantrone (Pix), a novel aza-anthracenadione recently approved by the European Medicines Agency in adult patients with multiply relapsed or refractory aggressive non-Hodgkin lymphoma (NHL). Etoposide and bendamustine were chosen as companion compounds due to available feasibility data in combination with Pix, as well as to their documented efficacy in salvage regimens in relapsed/refractory aggressive NHL and to a well-known feasibility profile when given alone or in combination. The monoclonal anti-CD20 antibody rituximab was added if tumor cells in the relapse biopsy specimen were CD20-positive. The adopted schedule consisted of Pixantrone 50 mg/m2 i.v. day 1+8, Etoposide 100 mg i.v. day 1, Bendamustine 90 mg i.v. day 1 with or without the addition of Rituximab 375 mg/m2 i.v. day 1 (PREBEN/PEBEN). If feasible, each cycle was given at 3-weekly intervals for a maximum of 6 cycles. All patients were assessed for chemosensitivity with PET/CT after cycle 1 or 2. G-CSF support was administered according to local guidelines. Results: A total of 8 evaluable patients with relapsed/refractory aggressive NHL were treated according to the PREBEN/PEBEN schedule.
summarizes the clinico-pathological features of the patient cohort along with selected feasibility and efficacy parameters related to the PREBEN/PEBEN regimen (PET/CT status after 1 or 2 courses):
| . | Patient characteristics . | PREBEN/PEBEN-related parameters . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pt # | Dx | Age | Sex | CS at relapse | N of prior Rx lines | Prior Tx | N courses | Tox grade 3-4 | Best response | DoR |
| 1 | DLBCL (ABC) | 70 | M | IV | 3 | N | 6 | thrombocytopenia | CR* | 6 mo |
| 2 | DLBCL (ABC) | 49 | M | IV | 3 | Y | 2 | - | PD | - |
| 3 | DLBCL (ABC) | 53 | M | IV | 2 | N | 2 | neutropenic fever | goodPR | 4+ mo |
| 4 | DLBCL (ABC) | 64 | M | IV | 2 | N | 2 | neutropenic fever | goodPR | 3+ mo |
| 5 | DLBCL (GCB) | 69 | F | IV | 3 | N | 2 | - | CR | 2+ mo |
| 6 | tFL | 62 | M | III | 3 | Y | 2 | neutropenic fever | SD | - |
| 7 | tCLL | 51 | F | IV | 5 | Y | 2 | neutropenic fever | PD | - |
| 8 | PTCL-NOS | 57 | F | IV | 2 | N | 6 | diarrhoea | CR | 4+ mo |
| . | Patient characteristics . | PREBEN/PEBEN-related parameters . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pt # | Dx | Age | Sex | CS at relapse | N of prior Rx lines | Prior Tx | N courses | Tox grade 3-4 | Best response | DoR |
| 1 | DLBCL (ABC) | 70 | M | IV | 3 | N | 6 | thrombocytopenia | CR* | 6 mo |
| 2 | DLBCL (ABC) | 49 | M | IV | 3 | Y | 2 | - | PD | - |
| 3 | DLBCL (ABC) | 53 | M | IV | 2 | N | 2 | neutropenic fever | goodPR | 4+ mo |
| 4 | DLBCL (ABC) | 64 | M | IV | 2 | N | 2 | neutropenic fever | goodPR | 3+ mo |
| 5 | DLBCL (GCB) | 69 | F | IV | 3 | N | 2 | - | CR | 2+ mo |
| 6 | tFL | 62 | M | III | 3 | Y | 2 | neutropenic fever | SD | - |
| 7 | tCLL | 51 | F | IV | 5 | Y | 2 | neutropenic fever | PD | - |
| 8 | PTCL-NOS | 57 | F | IV | 2 | N | 6 | diarrhoea | CR | 4+ mo |
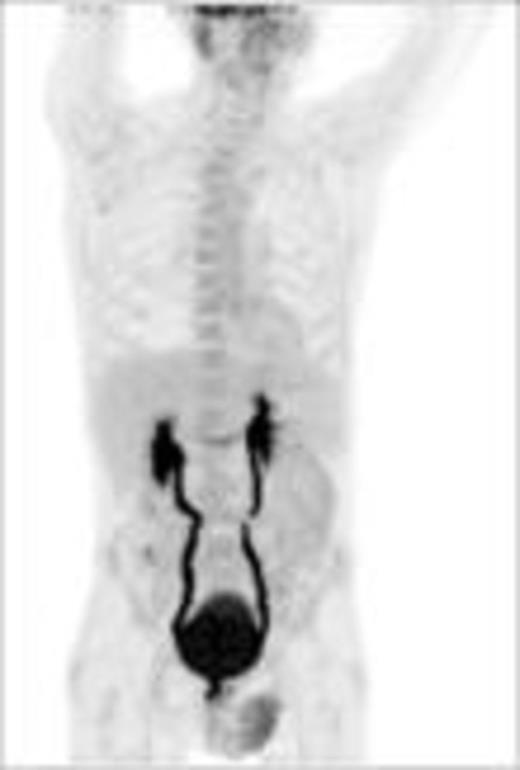
Baseline (pt#1) after 1 x PREBEN (pt#1)
Conclusion: The PREBEN/PEBEN schedule is feasible (out-patient regimen) and in individual patients it elicits profound responses early in the course of therapy. A phase 1-2 study in relapsed/refractory DLBCL and PTCL is in preparation.
d'Amore:Amgen: Research Funding; Sanofi Aventis: Research Funding; CTI Life Sciences: Advisory board, Advisory board Other, Speakers Bureau; Mundipharma: Advisory board, Advisory board Other, Speakers Bureau; Takeda: Advisory board, Advisory board Other, Speakers Bureau; Kyowa Kirin Pharmaceuticals: Advisory board Other, Speakers Bureau; Roche: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal