Abstract
Once thought to be a natural killer cell lymphoma, blastic plasmacytoid dendritic cell neoplasm, also termed CD4+CD56+ hematodermic neoplasm, is a rare clinical entity which derives from plasmacytoid dendritic cells. Cutaneous predominant (with better prognosis) and non-cutaneous subtypes (often accompanied by mediastinal involvement and a poorer prognosis) have been described. Many patients are treated with CHOP-like regimens, but the prognosis remains poor. Patients presenting with only skin lesions (median survival 21 months) had a better prognosis than patients presenting with both cutaneous and extra-cutaneous disease.
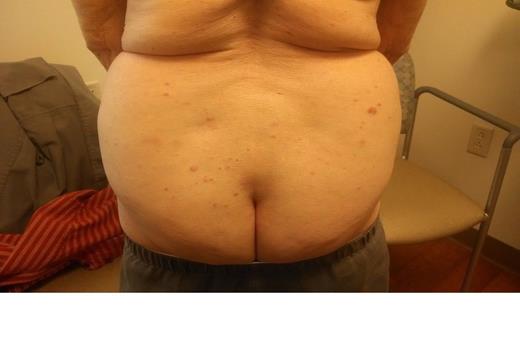
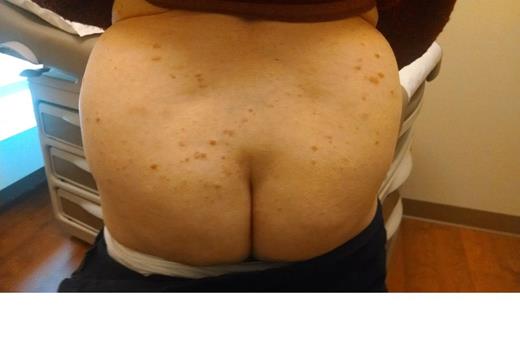
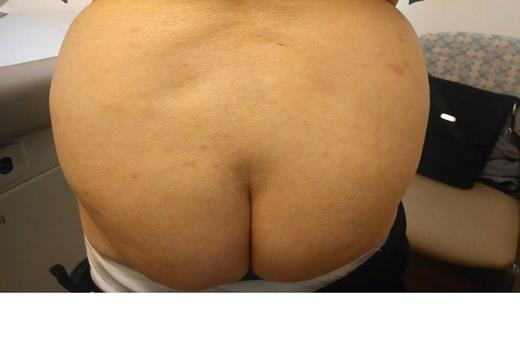
We describe an 86 yo female with a distant history of breast cancer. She noted rapid development of mildly itchy round pink cutaneous nodlues on the arms, buttock, and legs. She was treated with topical steroids without improvement. The CBC, calcium, and LDH were normal. ECOG 0. There was no adenopathy or organomegaly.
Skin biopsy demonstrated a dense lymphoid infiltrate involving the upper and mid dermis, as well as adnexal structures, without epidermotropism. The infiltrate was composed predominantly of large to intermediate sized lymphoid cells having moderate amounts of cytoplasm and oval to cleaved nuclei having open chromatin and one or more small nucleoli. Mitotic figures were present. The neoplastic cells expressed CD4, CD56. Ki-67 was expressed by many of the cells. The cells were negative for CD1a, CD3, CD5, CD7, CD8, PAX-5, CD20, CD79a, CD10, CD23, CD30, CD163, bcl-2, bcl-6, myeloperoxidase, kappa/lambda light chains, p63, Tdt, tryptase, chromogranin, MART-1, mammoglobin, CAM 5.2, and AE1/3. The morphology and immunophenotype was consistent with a CD4+/CD56+ hematodermic neoplasm.
The patient preferred no additional staging studies or therapy. Based on the available data, cutaneous-only disease was suggested. The disease was observed without intervention for a few weeks, but progressed on her skin. She then requested a gentle therapy that might provide palliative improvement and prevent progression.
Gemcitabine (2′,2′-difluorodeoxycytidine) is a well tolerated pyrimidine antimetabolite that impairs DNA synthesis and promotes induction of apoptosis. In patients with relapsed or refractory aggressive non-Hodgkin lymphoma, gemcitabine led to a 20% response rate with median survival of 6 months.
Therapy with Gemcitabine was administered at 1000 mg/M2 on days 1, 8, 15 every 28 days. There was rapid and marked improvement of the skin nodules. Treatment was very well tolerated with minimal bilateral leg edema. A 4 week chemotherapy holiday was associated with regrowth. Photographs document the disease progression before therapy and response of disease with ongoing therapy.
Gemcitabine may provide palliative benefit in some patients with blastic plasmacytoid dendritic cell neoplasm.
| Pre Therapy May 15 2014 . | . |
|---|---|
| Day 1 Chemotherapy May 23 2014 | |
| Day 22 Chemotherapy June 6 2014 |
| Pre Therapy May 15 2014 . | . |
|---|---|
| Day 1 Chemotherapy May 23 2014 | |
| Day 22 Chemotherapy June 6 2014 |
Off Label Use: Gemcitabine in blastic plasmacytoid dendritic cell neoplasm (CD4+CD56+ hematodermic neoplasm).
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal