Abstract
Background: Burkitt Lymphoma (BL) is the most common NHL in children and adolescents and has an excellent prognosis (≥80% 5years, EFS, Cairo et al. Blood, 2007). The prognosis has improved with the addition of targeted immunotherapy with rituximab (Goldman/Cairo et al, Leukemia, 2013, Cairo et al. JCO, 2012). However, a subset of patients with chemoimmunotherapy-resistant disease has a dismal prognosis (≤ 10% 5 years, EFS) (Miles/Cairo et al. BJH, 2012). Deregulation of signaling pathways controlled by protein phosphorylation underlies the pathogenesis of B-cell lymphomas, however, the extent to which they contribute to rituximab resistance is largely unknown (Barth et al. BJH, 2013). Obinutuzumab (GA101), a novel glycoengineered type II CD20 Ab, mediates enhanced cell death & ADCC against diffuse B-cell lymphoma vs. RTX (Mössner et al. Blood, 2010), and was recently approved by FDA and EMA for first line treatment of CLL in combination with chlorambucil.
Objective: To evaluate phosphorylation of signaling pathway proteins altered differentially after obinutuzumab or RTX treatment against RTX sensitive/ resistant BL cell lines.
Methods: Raji (CD20+, ATCC, Manhass, VA) and Raji-4RH (provided by M. Barth, Roswell Park Cancer Institute) were cultured in RPMI with 10% FBS. For in-vitro studies, tumor cells were incubated with 100 µg/ml obinutuzumab (supplied by Christian Klein, PhD, Roche Research & Early Development, Zurich), and/or RTX for 24 hrs . Cell death was evaluated by staining with AnnexinV/7AAD and analyzed by flow-cytometry. ADCC were performed with K562-IL-15-41BBL expanded NK cells at 20:1 effector: target ratio (E: T, n=3) using an europium release assay (Perkin-Elmer). For Phosphoproteomics analysis, we performed a mass spectrometry-based label-free quantitative phosphoproteomic profiling of the BL cell lines Raji, /Raji4RH in the presence/absence of obinutuzumab or rituximab (100µg/ml for 24h) or isotype control. Six milligrams of protein from each condition were digested by trypsin and peptides were subjected to phosphopeptide enrichment using metal oxide affinity chromatography (MOAC) and immunoprecipitation. An LTQ Orbitrap XL in-line with a Paradigm MS2 HPLC was employed for acquiring high-resolution MS and MS/MS data that were searched with the Swissprot Human taxonomic protein database.
Results: Obinutuzumab, compared to RTX, significantly enhanced cell death in Raji 45.1±3.3% vs. 32.7±6.8%, (p=0.005) & Raji4RH 15.8±2.2% vs. 2.1±1.5% (p=0.001), respectively. Overall survival of mice receiving 30 mg/kg of obinutuzumab was significantly increased when compared to mice receiving 30 mg/kg of RTX in Raji (p=0.05) & Raji4RH (p=0.024), respectively.
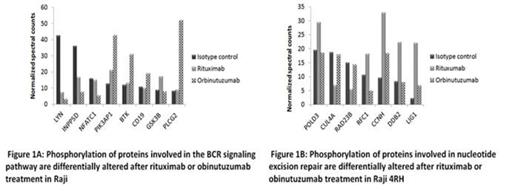
In Raji, 329 proteins were differentially phosphorylated (>1.5-fold change) between obinutuzumab vs. RTX. Of these proteins, 171 were expressed at higher levels in obinutuzumab than in RTX. Proteins differentially phosphorylated in response to obinutuzumab and RTX treatment were involved in the B-cell receptor (BCR) signaling pathway (LYN, BTK, CD19, PLCG2, INPP5D, NFATC1 and PIK3AP1), the spliceosome (TRA2A, DDX46 and PRPF31), and the cell cycle signaling pathway (WEE1, MMC3, GSK3B and CCNH). (Fig.1A)
Obinutuzumab and RTX also resulted in a differential phosphorylation of 606 proteins in Raji4RH. These proteins were involved in the spliceosome (CDC42, TRA2A and DDX42), tight junction (HCLS1, PRKCD, EPB41 and MYH2) and nucleotide excision repair (POLD3, CCNH and LIG1) pathways. (Fig.1B)
Differential phosphorylation of BCR signaling pathways proteins (BTK, PLCG2 and GSK3B) was validated by western blot studies after incubation with obinutuzumab vs. RTX in Raji/Raji4RH cell lines, reveled up regulation of BTK and PLCY2 after obinutuzumab treatment vs. RTX treatment in Raji BL cell line.
Conclusions: These data suggest that obinutuzumab vs. rituximab treatment result in global changes in BL proteins involved in BCR, spliceosome, cell cycle, nucleotide excision repair & tight junction signaling pathway. Furthermore, BCR signaling pathways appear more affected by obinutuzumab compared to RTX in Raji cell lines as compared to Raji4RH. Further, these data revealed the utility of unbiased phosphoproteome interrogation of obinutuzumab vs. rituximab mediated signaling events as well as characterizing signaling networks that may provide insights into pathogenetic mechanisms of rituximab resistance.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal