Abstract
Introduction
Nilotinib, a more potent and selective BCR-ABL-1 inhibitor than imatinib, is approved by the FDA and EMA 1) for the treatment of chronic and accelerated phase Philadelphia chromosome positive (Ph+) Chronic Myeloid Leukemia (CML) patients resistant or intolerant to prior therapy including imatinib and 2) for the treatment of newly-diagnosed Ph+ CML patients in the chronic phase (CML-CP). The aim of the present study was to evaluate the efficacy and safety of nilotinib in a Turkish population of newly-diagnosed Ph+ CML-CP patients.
Methods
The study was a multicenter, open-label, single-arm phase II clinical trial. All patients were to be treated with nilotinib (AMN107, Tasigna®) 300 mg BID for 24 months.
Results
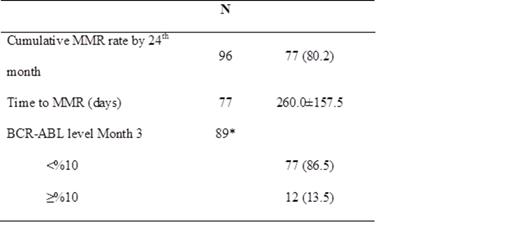
As of April 30, 2014, of the 96 patients out of a total 112 enrolled that had 24 month follow-up, 77 completed active treatment period. General characteristics of the patients at baseline are presented in Table 1.
Data are expressed as median (minimum-maximum) or number (%), where appropriate.
Molecular response by 24 months is presented in Table 2.
Data are presented as mean±standard deviation or number (%), where appropriate.
MMR (Major molecular rate): BCR-ABL/control gene ratio of ≤%0.1 measured by RQ-PCR as %
* The number of patients who were still on treatment at 3rd month.
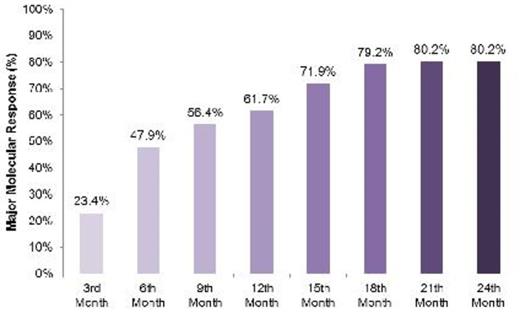
Cumulative MMR rates at various time points are shown in Figure 1.
Cumulative major molecular response rates at various time points
Cumulative major molecular response rates at various time points
A total of 486 adverse events (AEs) occurred; 93 AE led to discontinuation of the drug temporarily or permanently. Out of 96 patients 39 patients have temporally or permanently discontinued treatment. Permanent discontinuation rate due to adverse event was 9.6% (9 patients) which was the most common reason among all patients (19.8% - 19 patients) who permanently discontinued during 24 months. Thrombocytopenia was the most frequent (10.4%) AE, followed by hyperbilirubinemia (8.3%) and increased lipase level (7.3%) 19.8% of the patients entered in the trial had history of cardiovascular disease prior to trial entry and during the study cardiovascular events have been reported at 20.8% of patients. Despite published peripheral arterial occlusive disease (PAOD) reports related to nilotinib usage, there were no PAOD events reported at our cohort.
Conclusions
At 24 months, in Turkish patients with newly diagnosed CML, the cumulative MMR rate was 80.2%. Month 3 MMR rate was 86.5% and median time to MMR was 260.0±157.5 days. Out of 12 patients whose bcr-abl levels were above 10% at 3rd month, only 5 of them could reach to MMR by 24 months. Only one progression occurred, during the first year of therapy. These results suggest that high efficacy was achieved with nilotinib, an approved first-line therapy for newly diagnosed chronic phase of CML (CP-CML).
The results of the present study revealed that efficacy and safety of nilotinib in Turkish cohort are similar to those reported in ENESTnd. This study contribute to well established nilotinib profile for chronic phase CML patients which is a licensed alternative for the treatment of newly diagnosed Ph+ CML-CP in Turkey.
Saydam:Novartis Pharmaceuticals Corporation, Turkey: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees. Yavuz:Novartis Pharmaceuticals Corporation, Turkey: Membership on an entity's Board of Directors or advisory committees. Ali:Novartis Pharmaceuticals Corporation, Turkey: Membership on an entity's Board of Directors or advisory committees. Guvenc:Novartis Pharmaceuticals Corporation, Turkey: Membership on an entity's Board of Directors or advisory committees. Sonmez:Novartis Pharmaceuticals Corporation, Turkey: Membership on an entity's Board of Directors or advisory committees. Akkaynak:Novartis Pharmaceuticals Corporation, Turkey: Employment. Dag:Novartis Pharmaceuticals Corporation, Turkey: Employment.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal