Abstract
BACKGROUND:
The multiple myeloma (MM) represents a process in which an asymptomatic stage of monoclonal gammopathy of undetermined significance (MGUS) precedes virtually all cases of MM. It is known that tumor progression are determined by a favorable tumor microenvironment (TME) and in this scenario fibroblasts represent the principal cellular component in the TME.
A particular subpopulation of fibroblasts, cancer associated fibroblasts (CAFs), has recently raised the interest of many researchers due to their active participation in tumor growth and invasion and their association with higher malignancy grade, and poor prognosis. Recent findings indicate that the urokinase plasminogen activator (u-PA), and the urokinase receptor (u-PAR) are critical in cell invasion and degradation processes. Degradation and remodeling of the surrounding tissues are crucial in the early steps of tumor progression by facilitating expansion of the tumor mass, tumor cell proliferation, migration, and invasion. uPAR is expressed by multiple tumor associated cell types found in tumors. Targeting uPAR expressed on tumor-associated cells may be as important as targeting uPAR expressed on tumor cells and may lead to enhanced antitumor activity especially in those tumor types expressing uPAR on both types of cells.
METHODS:
Cell purification and cultures: BM mononuclear cells (BMMCs) were isolated by Ficoll-Hypaque gradient from heparinized bone marrow (BM) aspirates from 10 patients with relapse/refractory MM, 7 patients with asymptomatic MM, 7 with remission MM, 10 with MGUS. Fibroblasts were purified from BM stromal cells BMSCs through anti-fibroblasts-microbeads, and culture in DMEM medium with 10% FBS.
Cell phenotype analysis: CAFs were analyzed on heparinized bone marrow aspirates and they were identified by FSP1 and α-smooth muscle actin (α-SMA) expression on gated CD45-population. Expression of alpha-SMA in BM purified fibroblasts was also demonstrated by immunofluorescence staining.
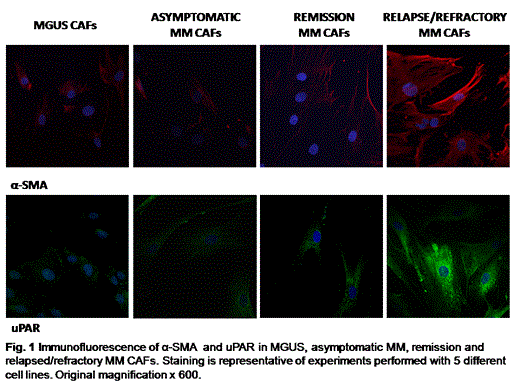
Immunofluorescence: CAFs were cultured in DMEM medium, fixed in paraformaldehyde, and permeabilized according to routine methods. The primary antibodies were anti–uPAR, and anti–α-SMA. Fibroblast nuclei were stained with DAPI.
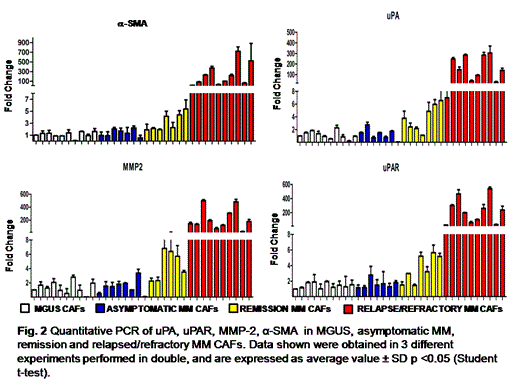
Quantitative PCR analysis: Complementary DNA was prepared from 1 ug total RNA using a GoScript reverse transcription system. The relative quantity of uPA, uPAR, MMP-2, α-SMA and vimentin messenger RNA were measured using the Applied Biosystems 7500 Fast Real-Time PCR System and determined by the comparative Ct method using 18S ribosomal RNA as the normalization gene.
The study was approved by the local Ethics Committee and all patients provided their informed consent in accordance with the Declaration of Helsinki.
RESULTS:
Cell phenotype analysis:Flow cytometry analysis showed that CAFs were increased in patients with relapse-MM compared to patients with asymptomatic, remission MM and MGUS suggesting that CAFs expansion is involved in MM progression.
CAFs activation: The increased frequency of alpha-SMA in CAFs of relapsed MM patients was demonstrated by the immunofluorescence analysis. Overall, these results suggest that MM activation is associated with the overexpression of uPAR. (Fig.1)
CAFs activation was also demonstrated by Real Time PCR and the figure 2 shows the overexpression of activation molecules as well as proinvasive systems in CAF of relapsed MM in comparison of MGUS and asymptomatic MM.
CONCLUSIONS:
In MM developement and progression the BM niche appears to play an important role in differentiation, migration, proliferation, and drug resistance of the malignant PCs.
The main goal of this proposal was to globally approach the expression of CAFs’ activation and proinvasive systems in the initiation and progression of MM.
Our results highlight an important mile stone on the phisiopatology of MM progression demonstrating that the CAF-activated phenotype is associated with an over-expression of the most important pro-invasive systems, and in particular the relapsed-MM CAFs seem to overexpress the fibrinolytic pattern of invasive tumor-like cells. On the basis of these results we aim to develop a biological model which can become a potential therapeutic target.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal