Abstract
Background: Noncoding RNAs play an important role in the pathogenesis of CLL. Recent publications suggested that higher miR-155 plasma levels (above 56th percentile) were associated with a significantly lower survival rate (P= 0.0122) in CLL patients treated with conventional treatments (Ferrajoli et al. Blood 2013;122:1891). Other studies revealed that expression of miR-29b or miR-181b significantly inhibits T cell leukemia/lymphoma 1(Tcl1) oncogene (Pekarsky et al. Cancer Res 2006;66:11590) whereas high Tcl1 expression correlates with aggressive CLL phenotype showing unmutated immunoglobulin variable region genes and ZAP70 positivity. The impact of these miR expressions in CLL patients undertaking allogeneic stem cell transplantation (alloSCT) is unknown.
Methods and Patients: We identified 41 patients with relapsed/refractory CLL who had received a non-myeloablative alloSCT at our center and in whom pre-transplant serum samples collected before initiating the patients’ allogeneic conditioning were available. We measured the serum expression of miR-155 as well as miR-15a/16-1 cluster, miR-29b and miR-181b. Total RNA was isolated from 100 µL of serum using the Total RNA Purification Kit. Serum miRNA levels were measured by qRT-PCR (TaqMan MicroRNA assays). The relative serum levels of miR-155, miR -15, miR-29b, miR-181b, and miR-16-1 were calculated using the equation 2−ΔCt, where ΔCt = mean CtmiRNA – mean Ctcel-miR-39, and Ct = threshold cycle. Twenty fmol of synthetic C. elegans miRNA (cel-miR-39) was spiked into each serum samples to normalize the experimental qRT-PCR data (cel-miR-39 Ct mean ± SD = 17.777± 0.628).
Forty one patients were initially evaluated. Thirteen of these 41 patients were later excluded as they received alemtuzumab for graft-versus-host disease (GVHD) prophylaxis. Therefore, our analysis was limited to 28 patients who received their alloSCT between 2000-2010. Median age (range) was 59 (45-70) years. Median number of prior therapies was 3 (range, 2-8). Eleven (39%) had a beta-2 microglobulin level of >3 mg/L. Ten of 12 (83%) patients with data that could be evaluated had unmutated immunoglobulin variable-region heavy-chain gene, and 4/19 (21%) had 17p13.1 deletion.
46% of patients had refractory disease at transplantation. The proportion of patients who received a matched related and a matched unrelated donor was 68% and 32%, respectively. All patients received non-myeloablative conditioning with fludarabine, cyclophosphamide, and rituximab as previously published (Khouri et al. Cancer 2011;117:4679). GVHD prophylaxis consisted of tacrolimus and methotrexate.
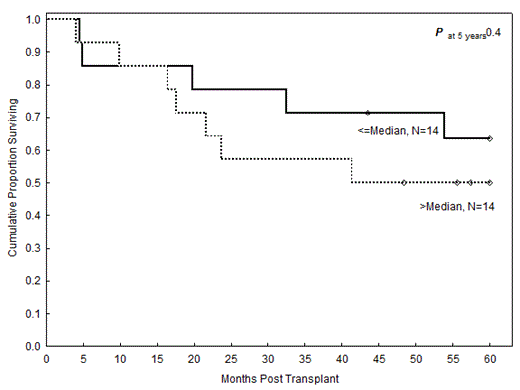
Results: Median (range) follow-up months was 68 (43-141). OS from the time of alloSCT was studied according to the relative expressions of miR under investigation (low, below median; high, above median). The 5-year OS rates of patients with low and high mir-155 were 63% and 50%, respectively (HR=1.6, P=0.4; Figure). No statistically significant differences were found in the other miRs studied. The 5-year OS was 57% for both the low and high miR-15a expression. The 5-year OS in low and high miR-29b and miR-181b were 52% and 61% (HR=0.8; P=0.7), and 60% and 53% (HR=1.1, P=0.9), respectively. The 5-year OS in low and high miR-16 were 43% and 71% (HR=0.4, P=0.2).
Conclusions: Our preliminary results suggest that serum levels of miR-155, miR -15, miR-29b, miR-181b, and miR-16 are not a statistically significant prognostic biomarker for survival in relapsed/refractory CLL undertaking alloSCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal