Abstract
Introduction:
Extracorporeal photopheresis (ECP) is a treatment modality that entails leukapheresis followed by mixing the buffy coat with 8-methoxypsoralen (8-MOP) and exposing it to UVA light. The buffy coat is then returned to the patient. ECP may be performed employing two different techniques: an on-line and off-line procedure. The off-line system includes two steps: a processing of buffy coat before reinfused to the patient.
The treatment is thought to have an immunomodulatory effect and is most commonly used to treat cutaneous T cell lymphoma, acute and chronic graft-versus-host disease (GVHD), and heart and lung allograft rejection. The exact mechanism and optimal cells dose to be treated is unknown. At the present time, a standard protocol is used generally without considering peripheral blood (PB) leukocytes counts or lymphocyte subpopulations (LP) of the patient. With the off-line system, PB leukocytes counts and LP analysis may be useful to choose the amount and distribution of cells to be infused.
The first objective of this study is to examine the quantitative correlation between PB LP and the buffy-coat in order to set individualized guidelines of treatment. Once this relationship is understood, the PB LP may serve as a surrogate marker for cell dose treated and help predicting the efficiency of ECP.
The second objetive of this study is to examine the mean performance of the buffy coat LP categorized according to PB leukocytes counts (<1.5 x 109/L and >1.5x 109/L).
Patients and methods: Twenty two consecutive patients with refractory GVHD were prospectively studied, from november 2009 to may 2014. Apheresis procedures were perfomed with COBE Spectra system (Terumo BCT®, Lakewood, CO, USA; version 7.0) by processing 1.5-2 times the patient blood volume. The product was transferred to a UVA-permeable bag (UVA, Macopharma, France), added 5 mL (0.1 mg) of 8-methoxypsoralen (8-MOP) aqueous solution (S.A.L.F.®, Cenate Sotto, Italy), exposed to UVA irradiation (Macogenic G2, Macopharma®), and then reinfused.
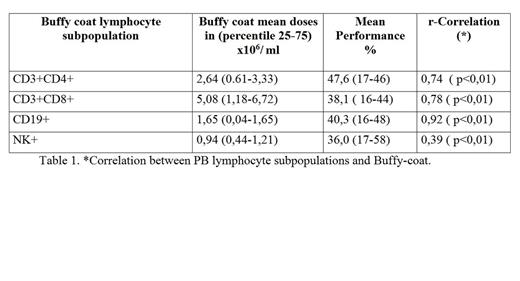
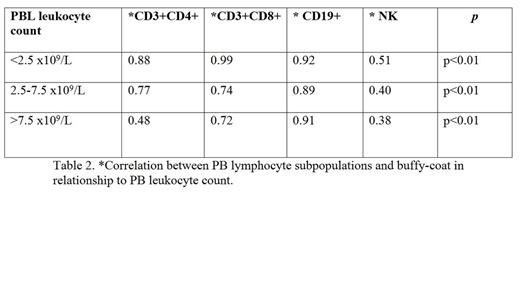
Peripheral blood sample was drawn before ECP. Just before reinfused, buffy coat sample was drawn. Spearman correlation analysis was performed between the LP CD3+CD4+, CD3+CD8+, CD19+, NK of the preapheresis peripheral blood patient and the buffy coat infused analyzed by multiparameter flow cytometry 5 colors (FC500-Beckman Coulter®) in the total sample and in three groups according to preapheresis leukocytes counts (<2.5, 2.5-7.5, >7.5x 109/L). The mean performance was calculated: CD3+CD4+, CD3+CD8+, CD19+, NK+ LP count in buffy coat/ CD3+CD4+, CD3+CD8+, CD19+, NK+ PB /ml x treatment volume (ml) x 100.
The mean performance of the buffy coat LP categorized according to preapheresis leukocytes counts (<1.5x 109/L and >1.5 109/L) were compared by Mann-Whitney test.
Results:
A total of 22 patients and 136 procedures were included in the final analysis. CD3+CD4+, CD3+CD8+, CD19+, NK LP in peripheral blood significantly correlated with those in buffy coat collected by COBE Spectra system, r= 0,74, 0.78, 0,92, 0,39 respectively (table 1). The LP mean doses and mean performance in buffy coat infused are specified in Table 1. The correlations was stronger in all LP with PB leukocytes counts <2.5 x 109/L (table2). We have not found any statistical correlation between the performance of the LP CD3+CD4+, CD3+CD8+, CD19+, NK according to PB leukocytes counts (<1.5x109/L and >1.5x109/L).
Conclusions:
The buffy coat contains great variability in lymphocyte subpopulations with predominant levels of CD3+CD8+. There is a robust linear relationship between all PB and buffy coat LP. The mean performance LP was around 40% and it was not related to very low PB leukocyte count (<1.5x109/L). The correlation was stronger with lower leukocytes counts in PB. If we could demonstrate a relationship between cell doses infused and clinical response, we could plan the necessary dose for each patient according to the PB leukocyte count and LP preapheresis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal