Abstract
Introduction
T-PLL is a mature post-thymic T-cell neoplasm with an aggressive clinical course (5-year overall survival 21%). Almost 75% of T-PLL cases harbor chromosome 14 translocations resulting in aberrant activation of the proto-oncogene TCL1A. Furthermore, in the majority of T-PLL cases the ATM gene is mutated or deleted, and recently it was reported that mutations in genes involved in the JAK-STAT pathway were found in 76% of T-PLL cases. Due to the rareness and aggressive nature of the disease, clinical trials are difficult to execute. By using a high-throughput ex vivo drug sensitivity and resistance testing (DSRT) platform covering 306 approved and investigational oncology drugs we systematically investigated the heterogeneity of drug responses in PLL-patients. As the impact of mutations on drug sensitivity is not well understood we aimed to identify relevant associations between the drug responses and genetic lesions in T-PLL patients.
Methods
Primary cells (MNCs) from seven T-PLL patients were obtained for drug screening. Samples were seeded in 384-well plates and 306 active substances were tested using a 10,000-fold concentration range resulting in a dose-response curve for each compound. Cell viability was measured after 72 h incubation and differential drug sensitivity scores (sDSS), representing leukemia-specific responses, were calculated by comparing patient samples to healthy donors. Hierarchical clustering of the drug responses was performed with Cluster 3.0 and Java Tree View. To assess the performance of the drug screening platform we also exchanged six samples with the German Cancer Research Center in Heidelberg for a comparison of results between two independent drug screening systems.
To understand heterogeneous pathway dependencies, drug sensitivities were correlated with somatic genetic variants and recurrent chromosomal aberrations. Genetic characterization was performed by exome sequencing of tumor and matched healthy cells to profile known recurrent genetic variants (ATM, STAT5b, IL2RG, JAK1, JAK3) as well as CNVs (TCL1A translocations, ATM deletions, recurrent chromosomal aberrations).
Results
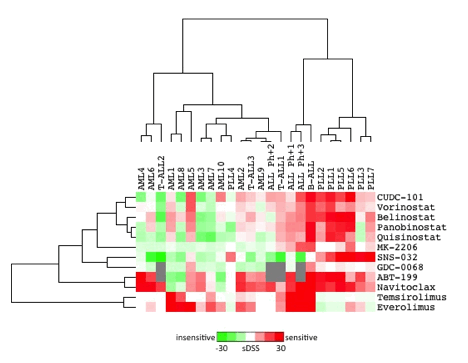
Four out of seven patient samples showed high sensitivity to small molecule BCL2 inhibitors navitoclax (IC50: 10-68nM) and ABT-199 (IC50: 14-45nM) and to HDAC inhibitors panobinostat and belinostat (IC50: 2-65nM). Intriguingly, the CDK inhibitor SNS-032 was effective in 6/7 patient samples (IC50: 7-95nM). SNS-032 inhibits Cdk2, Cdk7 and Cdk9, which control transcription of anti-apoptotic proteins including MCL1 and XIAP. As the AKT1/MTOR pathway is activated in many T-PLL patients due to expression of the TCL1A oncoprotein, it was interesting to observe that patient samples did not show any response to AKT inhibitors (MK-2206 and GDC-0068 IC50 values >1000 nM) nor to MTOR inhibitors (rapalogs temsirolimus and everolimus). Similarly, T-PLL cells were insensitive to JAK-inhibitors.
Clustering of drug responses from T-PLL patients with primary AML and ALL patient samples revealed the drug response profiles to be specific for T-PLL patients (Figure). 6/7 patients clustered together while the only patient (PLL4) in our cohort with confirmed mutations in the JAK-STAT pathway genes STAT5b (P702S) and IL2RG (K315E) exhibited a non-sensitive response pattern when compared to other samples (Figure). Interestingly, exome sequencing did not reveal any JAK mutations in our PLL-cohort (n=5) nor additional STAT5b or IL2RG mutations in other patients except in this unresponsive patient.
In the comparison between the platforms the correlation of the censored IC50 values from the 60 overlapping drugs was r=0.75. Similar fits of dose-response curves were seen for most drugs, although there were notable exceptions, which may be due to divergent culture conditions and day of read-out.
Conclusions
Ex vivo drug testing of primary patient cells has the potential to provide novel personalized drug candidates (such as BCL2, HDAC and CDK inhibitors) for T-PLL. The drug response pattern was T-PLL specific warranting further clinical testing. Drug screening, mutation analysis and RNA sequencing of additional patients is currently ongoing (n=20) to validate whether drug responses can be predicted based on the mutation profile or aberrant gene expression.
Clustering of T-PLL, AML and ALL patient samples based on DSRT results.
Kallioniemi:Medisapiens: Consultancy, Membership on an entity's Board of Directors or advisory committees. Porkka:Bristol-Myers Squibb: Honoraria, Research Funding; Novartis: Honoraria, Research Funding. Mustjoki:Bristol-Myers Squibb: Honoraria, Research Funding; Novartis: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal