Abstract
Background: Activating mutations in Notch receptors are found in multiple hematopoietic malignancies, including > 50% of patients with T-ALL, making it the most common genetic abnormality in this disease. GSIs block activation of Notch receptors and limit growth and survival in pre-clinical T-ALL models. However, various GSIs evaluated in clinical trials have had on target toxicities and have not been reported to show significant responses. CA216002 is a multicenter phase 1 trial designed to assess the safety of a novel intravenous GSI BMS-906024 in patients with relapsed or refractory T-ALL and T-cell lymphoblastic lymphoma (T-LL). We are presenting the initial toxicity profile and response data on this trial.
Methods: Adults with relapsed/refractory T-ALL or T-LL were enrolled and received BMS-906024 intravenously weekly at doses of 0.6 mg, 4 mg, and 6 mg. Due to the rapid progression of T-ALL in many cases, administration of glucocorticoids or other agents was permitted and dosing guidelines for dexamethasone were provided in the protocol.
Results: As of July 2, 2014, safety and response data are available on 25 patients (age 18-74 yrs) with relapsed/refractory T-ALL/T-LL that received at least one dose of BMS-906024, at doses of 0.6 mg (n=1), 4 mg (n=10), and 6 mg (n=14). Seven patients did not complete the first 4-week cycle due to rapid disease progression or disease-related death (n=5), infusion reaction (n=1), or an unrelated adverse event (n=1).
Safety: The drug-related grade 3-5 adverse events included grade 4 events of anemia, hypophosphatemia, and thrombocytopenia, and grade 3 events of diarrhea, febrile bone marrow aplasia, hepatotoxicity, hypophosphatemia, pancytopenia, and tumor lysis syndrome (n=1 each). Drug-related diarrhea was common (n=11, 44%), consistent with expectations for Notch inhibition, but was generally grade 1-2 with only one grade 3 event. One dose-limiting toxicity involving grade 3 elevations of ALT and AST without bilirubin elevation (reported as grade 3 hepatotoxicity) occurred at the 4 mg dose level. One death not related to disease progression occurred, due to GI and post-surgical hemorrhage associated with pancytopenia; hemorrhage was considered not related, but pancytopenia was considered related to study drug.
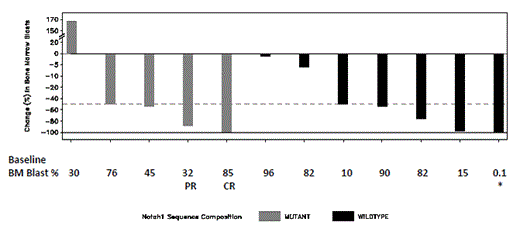
Responses: Eight patients (32%; 4 at 4 mg and 4 at 6 mg) had at least 50% reduction in bone marrow (BM) blasts, including one formal CR and one PR (both at 6 mg), and three of these eight had 98-100% clearance of BM blasts. (One patient, marked “*” below, began the study with 0.1% BM blasts and is not included in the eight.) The patient who achieved a CR began the study with 85% BM blasts and an absolute peripheral blood (PB) blast count of 38 k/mcL. By the end of the first cycle the BM and PB were cleared of blasts, and by the end of the second cycle there was count recovery. This patient received dexamethasone during the first cycle only, and left the study after 3 cycles in CR for an allogeneic transplant. The patient who achieved a PR began with 32% BM blasts, and by the end of the first cycle the BM blasts had decreased to 7% with improvement in ANC. The additional six patients with 50-100% decreases in BM blasts had residual lymphadenopathy, had incomplete count recovery or failed to meet other criteria which prevented them from being considered CR or PR based on the protocol definitions. One of these six patients also received hydroxyurea beginning on study day 16.
Biomarkers: The figure shows change in BM blasts in 12 patients with paired BM assessments and Notch mutation status available. BM responses occurred in both Notch mutant and wildtype patients.
Conclusions: Overall 8 of the 25 patients (32%) showed at least 50% reduction in BM blasts including one CR and one PR. Although the contribution of concurrent glucocorticoid therapy to the improvement in some patients is not clearly defined, the multiple responses on this trial suggest anti-leukemia activity of BMS-906024. This represents the first Notch targeting trial leading to multiple responses in relapsed/refractory T-ALL. BMS-906024 was relatively well tolerated, with minimal diarrhea in this population. The weekly dosing of this long-acting GSI shows promise for targeting Notch in T-ALL. Pharmacokinetic and additional biomarker data will be presented.
Zweidler-McKay:BMS: Research Funding. Off Label Use: BMS-906024 is in early Phase I clinical trials, and does not yet have an FDA-approved indication.. Douer:BMS: Research Funding. Ottmann:BMS: Consultancy, Honoraria, Research Funding. Vey:BMS: Honoraria. Thomas:BMS: Research Funding. Zhu:BMS: Employment. Huang:BMS: Employment, Equity Ownership. Bajaj:BMS: Employment. Fischer:BMS: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal