Key Points
Factor Xa activates PAR3 in the presence of EPCR by noncanonical cleavage at Arg41.
Noncanonical PAR3 activation induces Tie2 activation, upregulation and redistribution of ZO-1, and stabilization of tight junctions.
Abstract
Endothelial barrier protective effects of activated protein C (APC) require the endothelial protein C receptor (EPCR), protease-activated receptor (PAR) 1, and PAR3. In contrast, PAR1 and PAR3 activation by thrombin results in barrier disruption. Noncanonical PAR1 and PAR3 activation by APC vs canonical activation by thrombin provides an explanation for the functional selectivity of these proteases. Here we found that factor Xa (FXa) activated PAR1 at canonical Arg41 similar to thrombin but cleaved PAR3 at noncanonical Arg41 similar to APC. This unique PAR1-PAR3 activation profile permitted the identification of noncanonical PAR3 activation as a novel activation pathway for barrier protective tunica intima endothelial receptor tyrosine kinase 2 (Tie2). APC, FXa, and the noncanonical PAR3 tethered-ligand peptide induced prolonged activation of Tie2, whereas thrombin and the canonical PAR3 tethered-ligand peptide did not. Tie2 activation by FXa required PAR3 and EPCR. FXa and the noncanonical PAR3 tethered-ligand peptide induced Tie2- and PAR3-dependent upregulation of tight-junction-associated protein zona occludens 1 (ZO-1), translocation of ZO-1 to cell-cell borders, and the formation of typical ZO-1 honeycomb patterns that are indicative of tight-junction stabilization. These data provide intriguing novel insights into the diversification of functional selectivity of protease signaling achievable by canonical and noncanonical PAR activation, such as the activation of vascular-protective Tie2 by noncanonical PAR3 activation.
Introduction
Protease-activated receptors (PARs) are G protein-coupled receptors that comprise a subfamily of 4 receptors (PAR1, PAR2, PAR3, and PAR4). The PARs are unique in that they carry their own encrypted ligand encoded in the extracellular N-terminal tail. Proteolysis by coagulation or vascular proteases creates a new N-terminal tethered ligand that activates the PAR. Multiple proteases can activate PARs with each protease displaying a unique specificity for the different receptors.1 Efficient activation of PAR1 by thrombin is driven by binding of exosite I to the hirudin-like sequence of PAR1, thereby optimally positioning Arg41 in the active site of thrombin.2 Other proteases make use of coreceptors for efficient PAR activation. For instance, tissue factor permits PAR1 and PAR2 activation by the ternary complex, and the endothelial protein C receptor (EPCR) enhances activation of PAR1, PAR2, and PAR3 by activated protein C (APC).3-5
The requirement of PAR1 for APC’s cytoprotective effects created a conundrum because PAR1 activation by thrombin generally results in opposite proinflammatory and endothelial barrier disruptive effects.5,6 The discordant effects of PAR1 activation by thrombin vs APC are perhaps most apparent for the regulation of endothelial barrier function and vascular integrity, which raised the following question: how can activation of PAR1 by different proteases result in such remarkable opposite effects on endothelial barrier function? Activation of PAR1 by thrombin results in profound endothelial barrier disruptive effects that at least in part are mediated by activation of ras homolog gene family member A.7 In contrast, activation of PAR1 by APC results in endothelial barrier protective effects that involve β-arrestin-mediated activation of ras-related C3 botulinum toxin substrate 1 (Rac1) with possible contributions of tunica intima endothelial receptor tyrosine kinase 2 (Tie2) activation.8-11 Recently, novel insights revealed that thrombin and APC activate PAR1 at different cleavage sites. Thrombin activates PAR1 by proteolysis at canonical Arg41, whereas APC activates PAR1 by proteolysis at noncanonical Arg46.12 A synthetic peptide representing the PAR1 new N terminus after proteolysis by APC at Arg46 (NPNDKY…, aka TR47) stabilizes a subset of PAR1 conformations that preferentially employ biased β-arrestin-mediated signaling that results in activation of Rac1 and endothelial barrier protective effects.12
Additional explanations for divergent thrombin vs APC signaling may include the formation of PAR heterodimers involving transactivation and/or allosteric modulation of signaling. Recently, attention focused on PAR3 as both thrombin and APC can activate PAR3 and PAR3 forms a heterodimer with PAR1, thereby affecting the repertoire of G proteins that couple to PAR1.13 Functionally, PAR3 enhances PAR1-mediated endothelial barrier disruptive effects of thrombin but also contributes to neuroprotective effects of APC in ischemic stroke and cytoprotective effects of APC on podocytes.13-19 Analysis of PAR3 activation by APC and thrombin revealed that in the presence of EPCR, proteolysis of PAR3 by APC occurs at noncanonical Arg41, thereby generating the PAR3 tethered-ligand sequence GAPPNS… (aka P3R).19 Thrombin, on the other hand, cleaves PAR3 at canonical Lys38 generating the tethered-ligand sequence TFRGAP… (aka P3K).19 P3R but not P3K peptides mediate APC-like barrier protective effects in vitro and promote vascular integrity in vivo similar to the APC-derived PAR1 peptide TR47.19 Thus, PAR3 contributes to the diversification of signaling induced by thrombin vs APC. PAR3 is considered a nonsignaling receptor, and mechanisms for PAR3-mediated signaling remain incompletely understood. Endothelial barrier protective effects of P3R required PAR1 signaling implicating a role for PAR1-PAR3 heterodimers.19
In order to obtain additional insight into the relation between coagulation proteases with endothelial barrier protective effects and canonical/noncanonical PAR1 and PAR3 activation, the PAR proteolysis analysis was extended to factor Xa (FXa). Similar to APC, FXa mediates endothelial barrier protective effects that involve both PAR1, PAR2, and EPCR.20-22 To date, however, no potential role for PAR3 in FXa-induced barrier integrity has been implicated. Analysis of the FXa PAR1 and PAR3 proteolysis profiles revealed a unique crossover cleavage pattern between that of thrombin and APC. FXa activated PAR1 at canonical Arg41 similar to thrombin but cleaved PAR3 at noncanonical Arg41 similar to APC. This unique PAR1-PAR3 cleavage pattern of FXa provided a valuable tool to obtain novel insights into the contributions of canonical vs noncanonical PAR activation to the functional selectivity of protease signaling and to identify a novel pathway for the activation of Tie2 that is induced by noncanonical activation of PAR3.
Methods
See supplemental Methods (available on the Blood Web site) for details not found here.
PAR1 and PAR3 cleavage assays
Endothelial barrier assay
Endothelial cell permeability was measured in real time using the iCelligence system that determines changes in transendothelial electric resistance by electric cell-substrate impedance sensing (ECIS). Permeability of confluent endothelial cell (EA.hy926) layers after treatment with FXa (50 nM) or APC (50 nM) in the absence or presence of antibodies or inhibitors against PAR1, PAR3, EPCR, or Tie2 was determined using thrombin (2 nM), thrombin receptor activating peptide (TRAP) (10 µM), or histones (10 µg/mL) to induce endothelial permeability.
In-Cell Western
Assays were optimized for anti-pS1119-Tie2 as described with minor modifications using the In-Cell Western module of Odyssey Imager (LI-COR) with Imaging Studio Software, v3.0.24
Statistical analysis
Statistical significance (P < .05) was determined by 1-way ANOVA with Dunnett’s or Bonferroni’s multiple comparison test as appropriate (Prism 6.0; GraphPad Software, La Jolla, CA).
Results
PAR1 and PAR3 cleavage by FXa is dependent on EPCR
Cleavage of PAR1 and PAR3 by FXa was determined by the release of SEAP from SEAP-PAR1 and SEAP-PAR3 fusion constructs expressed in HEK-293 cells as previously described.12,19,25 FXa potently cleaved PAR1 in the presence but not in the absence of EPCR (Figure 1A). Cleavage of PAR1 was observed at FXa concentrations between 1 and 100 nM with half maximal cleavage at 27 nM FXa, which is similar to half-maximal cleavage of wt-SEAP-PAR1 by APC (23 nM) in the presence of EPCR but higher than that of thrombin (0.31 nM).12 Inhibition of FXa-mediated PAR1 cleavage by the FXa inhibitor rivaroxaban and by the FXa-blocking antibody (5051) but not by the nonblocking anti-FXa antibody (5050) indicated that PAR1 cleavage by FXa was specific for FXa and required its enzymatic activity (Figure 1B). Consistent with the requirement for EPCR, Gla-domainless βFXa failed to cleave PAR1, whereas the control βFXa cleaved PAR1 similar to wt-FXa.
EPCR-dependent PAR1 and PAR3 cleavage by FXa. FXa-mediated cleavage of PAR1 and PAR3 was analyzed by the proteolytic release of SEAP from SEAP-PAR1 and SEAP-PAR3 fusion protein expressed in HEK-293 cells in the absence or presence of wild-type (wt)-EPCR coexpression. (A,C) Dose response of PAR1 (A) and PAR3 (C) cleavage by FXa in the presence (□) or absence (▪) of EPCR. PAR1 and PAR3 cleavage was expressed as a percentage of the total available SEAP-PAR1 or SEAP-PAR3 on the cells. (B,D) Specificity controls of PAR1 and PAR3 cleavage by FXa on SEAP-PAR1/wt-EPCR (B) or SEAP-PAR3/wt-EPCR (D) cells. Inhibitors used were hirudin (20 U/mL) directed against thrombin and rivaroxaban (5 µM), blocking (5051) and nonblocking (5050) antibodies directed against FXa, and blocking antibody (C1) against APC (all 20 µg/mL). FXa, γ-carboxyglutamic acid (Gla)-domainless βFXa, or βFXa were used at 25 nM. (B, D) PAR1 and PAR3 cleavage was expressed relative to the cleavage in the absence of inhibitors or antibody. Data points represent mean ± standard error of the mean (SEM) of at least 3 independent experiments with 2 to 3 replicates per experiment.
EPCR-dependent PAR1 and PAR3 cleavage by FXa. FXa-mediated cleavage of PAR1 and PAR3 was analyzed by the proteolytic release of SEAP from SEAP-PAR1 and SEAP-PAR3 fusion protein expressed in HEK-293 cells in the absence or presence of wild-type (wt)-EPCR coexpression. (A,C) Dose response of PAR1 (A) and PAR3 (C) cleavage by FXa in the presence (□) or absence (▪) of EPCR. PAR1 and PAR3 cleavage was expressed as a percentage of the total available SEAP-PAR1 or SEAP-PAR3 on the cells. (B,D) Specificity controls of PAR1 and PAR3 cleavage by FXa on SEAP-PAR1/wt-EPCR (B) or SEAP-PAR3/wt-EPCR (D) cells. Inhibitors used were hirudin (20 U/mL) directed against thrombin and rivaroxaban (5 µM), blocking (5051) and nonblocking (5050) antibodies directed against FXa, and blocking antibody (C1) against APC (all 20 µg/mL). FXa, γ-carboxyglutamic acid (Gla)-domainless βFXa, or βFXa were used at 25 nM. (B, D) PAR1 and PAR3 cleavage was expressed relative to the cleavage in the absence of inhibitors or antibody. Data points represent mean ± standard error of the mean (SEM) of at least 3 independent experiments with 2 to 3 replicates per experiment.
To date, no information is available on whether FXa can activate PAR3. Incubation of SEAP-PAR3 cells with FXa showed only minimal PAR3 cleavage by FXa at concentrations >100 nM. However, PAR3 cleavage by FXa was greatly enhanced in the presence of EPCR (Figure 1C). Cleavage of PAR3 by FXa in the presence of EPCR was observed at FXa concentrations between 5 and 100 nM, which are comparable to APC concentrations required for efficient PAR3 cleavage.19 PAR3 cleavage by FXa was not attributable to trace amounts of thrombin or APC in the FXa preparation as neither hirudin nor a blocking APC antibody (C1 Ab) inhibited PAR3 cleavage by FXa (Figure 1D). Furthermore, PAR3 cleavage by FXa was inhibited by rivaroxaban and the blocking FXa antibody (5051) but not by the nonblocking anti-FXa antibody (5050), indicating that cleavage was specific for FXa’s enzymatic activity. No PAR3 cleavage was observed by Gla-domainless βFXa, indicating that the Gla domain of FXa was required for PAR3 cleavage consistent with the enhancement of PAR3 cleavage by FXa in the presence of EPCR.
Identification of the FXa cleavage site in PAR1 and PAR3
A synthetic peptide (P1, aka TR33-62) composed of PAR1 N-terminal tail residues 33 to 62 was used to identify the FXa cleavage site in PAR1. This approach led previously to the identification of noncanonical PAR1 cleavage by APC at Arg46.12 Cleavage of the P1 peptide by FXa generated 2 fragments that were similar to those generated by thrombin. The additional fragment generated by cleavage at Arg46 by APC was not observed with FXa, suggesting that FXa cleaved PAR1 at Arg41 similar to thrombin (Figure 2A). Half-maximal cleavage of the P1 peptide by FXa in 1 hour required ∼75 nM FXa (Figure 2B), whereas half-maximal cleavage of the P1 peptide by 25 nM FXa was achieved in ∼3 hours (Figure 2C). No APC-specific fragments derived from PAR1 cleavage at Arg46 were observed in the presence of FXa even when FXa was present at a high concentration or for a prolonged period of time (up to 10 hours), indicating that FXa did not cleave the P1 peptide at Arg46.
Identification of the PAR1 and PAR3 cleavage sites for FXa. Proteolysis of a synthetic PAR1 (P1: residues 33-62) and PAR3 (P3: residues 21-65) peptide by FXa, APC, and thrombin. (A) Chromatogram of P1 fragments generated in the absence (none) or presence of FXa (150 nM; 1 hour), thrombin (IIa) (10 nM; 1 hour), or APC (500 nM; 15 hours). (B) Concentration-dependent cleavage of the P1 peptide by FXa (1 hour). (C) Time-dependent cleavage of the P1 peptide by FXa (25 nM). (D) Chromatogram of the P3 fragments generated by FXa (150 nM; 1 hour) and thrombin (10 nM; 1 hour). Peptide P3 and the C-terminal fragments after cleavage at Lys38 (P3K) or Arg41 (P3R) are indicated for reference. (E) Concentration-dependent cleavage of the P3 peptide by FXa (10 hours). (F) Time-dependent cleavage of the P3 peptide by FXa (150 nM). (A, D) Representative chromatograms. (B-C,E-F) Data points represent the mean ± standard deviation (SD) of 3 independent experiments.
Identification of the PAR1 and PAR3 cleavage sites for FXa. Proteolysis of a synthetic PAR1 (P1: residues 33-62) and PAR3 (P3: residues 21-65) peptide by FXa, APC, and thrombin. (A) Chromatogram of P1 fragments generated in the absence (none) or presence of FXa (150 nM; 1 hour), thrombin (IIa) (10 nM; 1 hour), or APC (500 nM; 15 hours). (B) Concentration-dependent cleavage of the P1 peptide by FXa (1 hour). (C) Time-dependent cleavage of the P1 peptide by FXa (25 nM). (D) Chromatogram of the P3 fragments generated by FXa (150 nM; 1 hour) and thrombin (10 nM; 1 hour). Peptide P3 and the C-terminal fragments after cleavage at Lys38 (P3K) or Arg41 (P3R) are indicated for reference. (E) Concentration-dependent cleavage of the P3 peptide by FXa (10 hours). (F) Time-dependent cleavage of the P3 peptide by FXa (150 nM). (A, D) Representative chromatograms. (B-C,E-F) Data points represent the mean ± standard deviation (SD) of 3 independent experiments.
Cleavage of a synthetic PAR3 N-terminal tail peptide (P3) composed of PAR3 residues 21 to 65 was used to identify the FXa cleavage site in PAR3.19 Remarkably, incubation of the P3 peptide with FXa generated 2 fragments that were different from those generated by thrombin (Figure 2D). Chromatography of synthetic peptides representing the C-terminal fragment after cleavage by APC (P3R; residues 42-65) or thrombin (P3K; residues 39-65) indicated that the C-terminal fragment of P3 generated by FXa was identical to the peak corresponding to the P3R fragment, suggesting that FXa cleaved PAR3 at noncanonical Arg41 similar to APC. Half-maximal cleavage of the P3 peptide in 10 hours required ∼50 nM FXa (Figure 2E), whereas half-maximal cleavage by 150 nM FXa was achieved in ∼3 hours (Figure 2F). Importantly, no evidence of P3 cleavage by FXa at the Lys38 thrombin cleavage site was observed.
Canonical and noncanonical cleavage of PAR1 and PAR3 by FXa on cells
SEAP-PAR1 and SEAP-PAR3 containing cleavage site mutations were used to confirm FXa-mediated PAR1 and PAR3 cleavage at Arg41 on cells. In the presence of EPCR, FXa readily cleaved Arg46Gln-SEAP-PAR1 (Figure 3A). In contrast, no FXa-mediated cleavage of PAR1 was observed when either Arg41 or both Arg41 and Arg46 were mutated. Furthermore, no FXa-mediated PAR1 cleavage was observed in the absence of EPCR (Figure 3B). Thus, FXa cleaved PAR1 at canonical Arg41 on cells, and this cleavage required EPCR.
Proteolysis of PAR1 and PAR3 cleavage site mutants by FXa on cells. Cleavage of SEAP-PAR1 (A-B) and SEAP-PAR3 (C-D) mutants by FXa in the presence (A,C) and absence (B,D) of wt-EPCR. SEAP released in the media was determined 1 hour after the addition of FXa and corrected for background and spontaneous SEAP release in the absence of protease (∼2%). The activity of released SEAP was expressed as a percentage of total available SEAP activity on the cells. No SEAP activity was detected on HEK-293 or wt-EPCR HEK-293 cells in the absence of SEAP-PAR1 or SEAP-PAR3. Data points represent mean ± SEM of at least 3 independent experiments with 2 to 3 replicates per experiment. K, Lys; Q, Gln; R, Arg.
Proteolysis of PAR1 and PAR3 cleavage site mutants by FXa on cells. Cleavage of SEAP-PAR1 (A-B) and SEAP-PAR3 (C-D) mutants by FXa in the presence (A,C) and absence (B,D) of wt-EPCR. SEAP released in the media was determined 1 hour after the addition of FXa and corrected for background and spontaneous SEAP release in the absence of protease (∼2%). The activity of released SEAP was expressed as a percentage of total available SEAP activity on the cells. No SEAP activity was detected on HEK-293 or wt-EPCR HEK-293 cells in the absence of SEAP-PAR1 or SEAP-PAR3. Data points represent mean ± SEM of at least 3 independent experiments with 2 to 3 replicates per experiment. K, Lys; Q, Gln; R, Arg.
In PAR3, mutation of the noncanonical Arg41 cleavage site greatly diminished PAR3 cleavage by FXa, whereas mutation of the canonical Lys38 cleavage site had little effect on FXa-mediated PAR3 cleavage (Figure 3C). Mutation of both Lys38 and Arg41 abolished PAR3 cleavage by FXa, indicating that FXa cleaved PAR3 at Arg41 similar to APC. The presence of EPCR was required for noncanonical PAR3 cleavage at Arg41 because no PAR3 cleavage was observed in the absence of EPCR (Figure 3D).
Taken together, these data demonstrate that FXa cleaved PAR1 at Arg41 similar to thrombin and not at Arg46 like APC, whereas FXa cleaved PAR3 at the noncanonical Arg41 similar to APC but not at the canonical Lys38 corresponding to cleavage by thrombin.12,19 Thus, the EPCR-dependent canonical PAR1 and noncanonical PAR3 proteolysis profile of FXa is a crossover between that of thrombin and APC.
FXa-mediated changes in endothelial barrier integrity
Similar to APC, FXa was shown to reduce thrombin-induced permeability of endothelial cells as determined by the leakage of Evans blue/bovine serum albumin through endothelial cell monolayers.20-22 To understand how PAR1 and PAR3 cleavage at Arg41 contributes to FXa’s effects on barrier function, changes in ECIS using the iCelligence system were monitored in EA.hy926 cells. This system provides the advantage of real-time monitoring of the disruption and recovery of the endothelial barrier function (supplemental Figure 1). Surprisingly, FXa (50 nM) induced an immediate drop in the cell index of ∼60%, indicating that FXa induced a loss of cell barrier function (Figure 4A). The acute decrease in the cell index was comparable to that induced by thrombin (2 nM) (Figure 4B), whereas APC did not induce a similar immediate decrease in the cell index (Figure 4A-B), suggesting that PAR1 activation at Arg41 was potentially responsible for the immediate loss of cell barrier function upon contact of FXa with the cells.
Endothelial barrier regulation by FXa. Effects of thrombin, APC, and FXa on endothelial barrier function by monitoring real-time changes in transendothelial electric resistance by ECIS in EA.hy926 cells. (A) Kinetics of the changes in cell index after addition of APC (25 nM) or FXa (25 nM). Cell index was normalized against nontreated cells (not shown). Arrow indicates the addition of APC or FXa. (B) Quantification of early effects on endothelial barrier function by FXa (25 nM), APC (25 nM), and thrombin (IIa) (2 nM). (C) Kinetics of the changes in cell index cells in the presence and absence of FXa (25 nM; 3 hours) induced by TRAP (TFFLRN; 10 µM). Arrow indicates the addition of TRAP. (D) Quantification of inhibition of TRAP-induced (TFLLRN; 10 µM) endothelial barrier permeability by FXa (25 nM) and APC (25 nM). (E) Time-dependent protection of endothelial barrier integrity by FXa (25 nM) against thrombin-induced (2 nM) permeability. (F) Protection of endothelial barrier function by APC (25 nM) or FXa (25 nM) against permeability induced by histones (10 µg/mL). (G) The effects of blocking PAR1 (WEDE15/ATAP2; 25 µg/mL), PAR2 (SAM11; 25 µg/mL), and PAR3 (H103/Moab19b; 25 µg/mL) antibodies on early FXa-induced (25 nM) endothelial barrier disruption. (H) The effect of blocking PAR1, PAR2, and PAR3 antibodies on protection of endothelial barrier integrity by FXa (25 nM) against barrier disruption induced by TFLLRN (10 µM). (I) The effects of blocking PAR1, PAR2, and PAR3 antibodies on protection of endothelial barrier integrity by FXa (25 nM) against barrier disruption induced by SFLLRN (10 µM). (J) FXa-mediated (25 nM; 3 hours) endothelial barrier protection in the presence of blocking antibodies against PAR3. Endothelial permeability was induced by thrombin (2 nM). The maximal permeability induced by thrombin in anti-PAR3 antibodies treated cells was considered as 100%. (D-F,H-I) Maximal permeability induced by TRAP, thrombin, or histones in nontreated cells was taken as 100%. (J) Maximal permeability induced by thrombin in the presence of blocking PAR3 antibodies was taken as 100%. (G-I) Statistical analysis of effects compared with FXa. (A-J) Data points represent mean ± SD of at least 3 independent experiments. *P < .05; **P < .01; ****P < .0001. ns, not significant.
Endothelial barrier regulation by FXa. Effects of thrombin, APC, and FXa on endothelial barrier function by monitoring real-time changes in transendothelial electric resistance by ECIS in EA.hy926 cells. (A) Kinetics of the changes in cell index after addition of APC (25 nM) or FXa (25 nM). Cell index was normalized against nontreated cells (not shown). Arrow indicates the addition of APC or FXa. (B) Quantification of early effects on endothelial barrier function by FXa (25 nM), APC (25 nM), and thrombin (IIa) (2 nM). (C) Kinetics of the changes in cell index cells in the presence and absence of FXa (25 nM; 3 hours) induced by TRAP (TFFLRN; 10 µM). Arrow indicates the addition of TRAP. (D) Quantification of inhibition of TRAP-induced (TFLLRN; 10 µM) endothelial barrier permeability by FXa (25 nM) and APC (25 nM). (E) Time-dependent protection of endothelial barrier integrity by FXa (25 nM) against thrombin-induced (2 nM) permeability. (F) Protection of endothelial barrier function by APC (25 nM) or FXa (25 nM) against permeability induced by histones (10 µg/mL). (G) The effects of blocking PAR1 (WEDE15/ATAP2; 25 µg/mL), PAR2 (SAM11; 25 µg/mL), and PAR3 (H103/Moab19b; 25 µg/mL) antibodies on early FXa-induced (25 nM) endothelial barrier disruption. (H) The effect of blocking PAR1, PAR2, and PAR3 antibodies on protection of endothelial barrier integrity by FXa (25 nM) against barrier disruption induced by TFLLRN (10 µM). (I) The effects of blocking PAR1, PAR2, and PAR3 antibodies on protection of endothelial barrier integrity by FXa (25 nM) against barrier disruption induced by SFLLRN (10 µM). (J) FXa-mediated (25 nM; 3 hours) endothelial barrier protection in the presence of blocking antibodies against PAR3. Endothelial permeability was induced by thrombin (2 nM). The maximal permeability induced by thrombin in anti-PAR3 antibodies treated cells was considered as 100%. (D-F,H-I) Maximal permeability induced by TRAP, thrombin, or histones in nontreated cells was taken as 100%. (J) Maximal permeability induced by thrombin in the presence of blocking PAR3 antibodies was taken as 100%. (G-I) Statistical analysis of effects compared with FXa. (A-J) Data points represent mean ± SD of at least 3 independent experiments. *P < .05; **P < .01; ****P < .0001. ns, not significant.
Notwithstanding, after incubation of FXa with endothelial cells for 3 hours, FXa substantially protected (∼40%) against TRAP-induced loss of barrier function (Figure 4C), confirming the barrier protective effects of FXa.3,4,20-22 FXa-mediated endothelial barrier protective effects were of similar magnitude to those induced by APC (Figure 4D). The protective effects by FXa were time dependent, and the most effective barrier protection was observed after 2 to 3 hours (Figure 4E). Both FXa and APC also blunted histone-induced permeability (Figure 4F). Histones were used to induce barrier disruption independently of PAR1 because FXa cleaved PAR1 at Arg41 and PAR1 desensitization can potentially give a false impression of barrier protection when a PAR1-dependent protease, such as thrombin, is used to induce barrier disruption. Furthermore, the amidolytic activity of FXa and thrombin on cells was lost within ∼1 hour, and barrier disruption induced by the PAR1 tethered-ligand peptide TRAP was indistinguishable between cells that were incubated for 3 hours with thrombin (2 nM) and naïve endothelial cells (supplemental Figure 2). Thus, FXa-mediated endothelial barrier protective effects were not attributable to PAR1 desensitization.
Blocking antibodies were used to address the individual contributions of PAR1, PAR2, and PAR3 to FXa-mediated barrier function. The acute barrier disruption induced by FXa (Figure 4B) was prevented by PAR1 blocking antibodies, whereas PAR2 and PAR3 blocking antibodies had only a small effect (<10%) (Figure 4G). Thus, acute loss of barrier integrity by FXa was mediated by PAR1 activation at Arg41. In order to determine contributions of PAR1 activation to FXa-mediated barrier protection, TRAP peptides were used to induce barrier disruption as PAR1 blocking antibodies prevented the use of thrombin for this purpose. Previous studies implicated both PAR1 and PAR2 for FXa-mediated barrier protective signaling responses in endothelial cells.20-22 However, FXa-induced endothelial barrier protection required PAR1 but not PAR2 when barrier disruption was induced by the high affinity “TFLLRN…” TRAP peptide26 that induced robust barrier disruption (Figure 4H). In contrast, FXa-induced endothelial barrier protection was dependent on both PAR1 and PAR2 when barrier disruption was induced by the natural “SFLLRN…” TRAP tethered ligand (Figure 4I), in agreement with previous reports.20-22 Blocking antibodies against PAR3 reduced thrombin-mediated barrier disruption by ∼15% (Figure 4J), confirming that canonical activation of PAR3 enhanced barrier disruptive effects of thrombin.13,16,19 However, PAR3 blocking antibodies also partially but significantly reduced the barrier protective effect of FXa. Thus, noncanonical PAR3 activation contributed to the endothelial barrier protective effects of FXa.
Modulation of PAR1-mediated signaling by PAR3
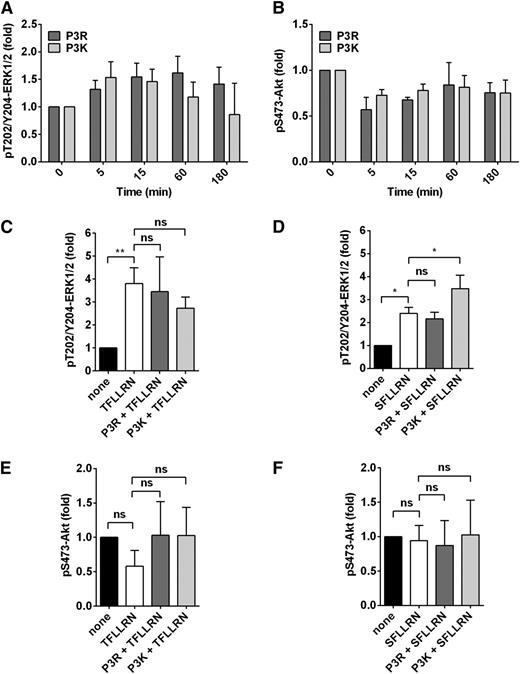
The endothelial barrier protective proteases FXa and APC both induce noncanonical PAR3 activation at Arg41, and synthetic peptides representing the tethered-ligand sequence of PAR3 after noncanonical cleavage induce barrier protective effects in vitro and vascular integrity in vivo.19 However, signaling mechanisms employed by PAR3 remain undefined. Therefore, the canonical (P3K) and noncanonical (P3R) PAR3 tethered-ligand peptides were analyzed for their effects on the important signaling nodes extracellular signal-regulated kinase (ERK) 1/2 and Akt, which were previously shown to be selectively induced by canonical and noncanonical PAR1 tethered-ligand peptides.12
Neither P3K nor P3R peptides directly induced significant phosphorylation of Thr202/Tyr204-ERK1/2 (Figure 5A) or Ser473-Akt (Figure 5B) in endothelial cells. Next, modulation of PAR1-mediated signaling by PAR3 peptides was determined using the TRAP peptides (TFLLRN or SFLLRN). No effects of P3R peptide were observed on ERK1/2 phosphorylation induced by TFLLRN (Figure 5C) or SFLLRN (Figure 5D). Interestingly, the canonical P3K peptide enhanced ERK1/2 phosphorylation induced by SFLLRN (Figure 5D) but not by TFLLRN (Figure 5C). These results are consistent with the contribution of PAR3 to thrombin-induced barrier disruption.13,16,19
Modulation of PAR1-dependent ERK1/2 signaling by PAR3 in endothelial cells. Phosphorylation of Thr202/Tyr204-ERK1/2 and Ser473-Akt by P3R (residues 42-54) and P3K (residues 39-54) was determined in the absence and presence of TRAP-peptides TFLLRN or SFLLRN. (A) Time-dependent ERK1/2 phosphorylation by P3R (50 µM) and P3K (50 µM). (B) Phosphorylation of Akt by P3R (50 µM) and P3K (50 µM) over time. (C-D) ERK1/2 phosphorylation at 15 minutes induced by TFLLRN (25 µM) (C) or SFLLRN (25 µM) (D) after pretreatment (1 hour) with P3R (50 µM) or P3K (50 µM). (E, F) Akt phosphorylation at 15 minutes induced by TFLLRN (25 µM) (E) or SFLLRN (25 µM) (F) after pretreatment (1 hour) with P3R (50 µM) or P3K (50 µM). (A-F) Data points represent mean ± SD of at least 3 independent experiments. *P < .05; **P < .01. ns, not significant.
Modulation of PAR1-dependent ERK1/2 signaling by PAR3 in endothelial cells. Phosphorylation of Thr202/Tyr204-ERK1/2 and Ser473-Akt by P3R (residues 42-54) and P3K (residues 39-54) was determined in the absence and presence of TRAP-peptides TFLLRN or SFLLRN. (A) Time-dependent ERK1/2 phosphorylation by P3R (50 µM) and P3K (50 µM). (B) Phosphorylation of Akt by P3R (50 µM) and P3K (50 µM) over time. (C-D) ERK1/2 phosphorylation at 15 minutes induced by TFLLRN (25 µM) (C) or SFLLRN (25 µM) (D) after pretreatment (1 hour) with P3R (50 µM) or P3K (50 µM). (E, F) Akt phosphorylation at 15 minutes induced by TFLLRN (25 µM) (E) or SFLLRN (25 µM) (F) after pretreatment (1 hour) with P3R (50 µM) or P3K (50 µM). (A-F) Data points represent mean ± SD of at least 3 independent experiments. *P < .05; **P < .01. ns, not significant.
Finally, no Akt phosphorylation at Ser473 was observed in endothelial cells treated with TFLLRN (Figure 5E) or SFLLRN (Figure 5F) in the presence of either P3K or P3R. This suggested that the noncanonical P3R peptide did not bias PAR1 to TRAP-mediated Akt signaling comparable to that induced by the noncanonical TR47 peptide that represents the PAR1 tethered ligand after cleavage at Arg46 by APC. Thus, the noncanonical P3R peptide presumably employs other signaling pathways to mediate endothelial barrier protection effects.
Noncanonical PAR3 activation induces Tie2 activation
The endothelial cell–specific receptor tyrosine kinase Tie2 provides important contributions to the regulation of endothelial barrier function, and transactivation of Tie2 by APC was implicated to contribute to improved barrier function.10,27,28 Therefore, the effects of FXa on Tie2 activation were determined. FXa caused a rapid activation of Tie2 as reflected by phosphorylation at Ser1119 (Figure 6A) and Y992 (supplemental Figure 3). Maximal activation of Tie2 by FXa was achieved after ∼60 minutes and was sustained for up to 3 hours. In contrast, Tie2 activation by thrombin was fast and transient, reaching maximal levels after ∼5 minutes and returning to undetectable baseline levels within 1 hour. Tie2 activation by APC was both fast and sustained.
FXa induces PAR3-dependent phosphorylation of Tie2 in endothelial cells. Activation of Tie2 was determined by analysis of Tie2 phosphorylation at Ser1119-Tie2. (A) Time-dependent Tie2 phosphorylation by FXa (50 nM), APC (50 nM), and thrombin (IIa) (2 nM). (B) FXa (50 nM) induced phosphorylation of Tie2 at 90 minutes after pretreatment with antibodies against Tie2 (anti-hTie2; 25 µg/mL), PAR1 (WEDE15/ATAP; 25 µg/mL), PAR3 (Moab19b; 25 µg/mL), EPCR (rcr-252; 25 µg/mL), or an inhibitor against PAR1 (vorapaxar; 1 µM). (C) Induction of Tie2 activation at 15 and 90 minutes by the tethered-ligand peptides of PAR1, PAR2, and PAR3: TRAP (SFLLRN or TFLLRN), TR47 (PAR1 residues 47-66), SLIGKV (PAR2), and P3R (42-65). (D) Concentration-dependent Tie2 phosphorylation by P3R (42-65) and P3K (39-65). Insert shows an expanded x-axis between 0 and 1.5 µM peptide. (E) Time-dependent phosphorylation of Tie2 by P3R (42-65) (50 µM) and P3K (39-65) (50 µM). (A-E) Data points represent the mean ± SEM of at least 3 independent experiments with 3 replicates per experiment. *P < .05; **P < .01; ***P < .001; ****P < .0001.
FXa induces PAR3-dependent phosphorylation of Tie2 in endothelial cells. Activation of Tie2 was determined by analysis of Tie2 phosphorylation at Ser1119-Tie2. (A) Time-dependent Tie2 phosphorylation by FXa (50 nM), APC (50 nM), and thrombin (IIa) (2 nM). (B) FXa (50 nM) induced phosphorylation of Tie2 at 90 minutes after pretreatment with antibodies against Tie2 (anti-hTie2; 25 µg/mL), PAR1 (WEDE15/ATAP; 25 µg/mL), PAR3 (Moab19b; 25 µg/mL), EPCR (rcr-252; 25 µg/mL), or an inhibitor against PAR1 (vorapaxar; 1 µM). (C) Induction of Tie2 activation at 15 and 90 minutes by the tethered-ligand peptides of PAR1, PAR2, and PAR3: TRAP (SFLLRN or TFLLRN), TR47 (PAR1 residues 47-66), SLIGKV (PAR2), and P3R (42-65). (D) Concentration-dependent Tie2 phosphorylation by P3R (42-65) and P3K (39-65). Insert shows an expanded x-axis between 0 and 1.5 µM peptide. (E) Time-dependent phosphorylation of Tie2 by P3R (42-65) (50 µM) and P3K (39-65) (50 µM). (A-E) Data points represent the mean ± SEM of at least 3 independent experiments with 3 replicates per experiment. *P < .05; **P < .01; ***P < .001; ****P < .0001.
The sustained Tie2 activation induced by FXa and APC prompted investigations into the role of PAR3 in Tie2 activation because both proteases induce noncanonical PAR3 activation. Antibodies blocking PAR3 proteolytic activation (combination of H103 and Mab19b19,29 ) prevented FXa-induced Tie2 phosphorylation at Ser1119 (Figure 6B) and at Y992 (supplemental Figure 3). Similarly, EPCR blocking antibodies inhibited FXa-induced phosphorylation of Tie2 consistent with the observation that PAR3 activation at Arg41 by FXa required EPCR (Figure 1C). Antibodies that block the activation of PAR1 (WEDE15 and ATAP2) or the PAR1 antagonist vorapaxar, on the other hand, inhibited Tie2 activation by ∼50%, indicating that PAR1 contributed to Tie2 activation but was not required. No FXa-mediated Tie2 phosphorylation was observed in presence of a Tie2 activity-blocking antibody, confirming that the pS1119-Tie2 signal was specific (Figure 6B).
Agonist peptides were used to identify the contribution of activation of PAR1, PAR2, and PAR3 to Tie2 activation. Remarkably, neither the PAR1 agonist peptides (canonical TRAP [TFLLRN or SFLLRN] or noncanonical TR47) nor the PAR2 agonist peptide SLIGKV induced Tie2 activation (Figure 6C). In contrast, the noncanonical PAR3 peptide P3R induced robust Tie2 activation. To determine whether activation of Tie2 was specific for noncanonical activation of PAR3, the dose response and time course of Tie2 activation induced by canonical (P3K) and noncanonical (P3R) PAR3 tethered-ligand peptides were determined. P3R induced potent activation of Tie2 achieving maximal activation at ∼0.8 µM P3R (Figure 6D). In contrast, P3K failed to induce Tie2 activation at low concentration, and induction of Tie2 activation comparable to half-maximal induction by P3R required a 30-fold higher concentration of P3K. Tie2 activation by P3R was relatively fast and reached half-maximal activation in 5 minutes, whereas Tie2 activation by P3K was 10-fold slower (Figure 6E). Importantly, these data indicated that Tie2 activation was selectively induced by noncanonical PAR3 activation.
Stabilization of tight junctions by noncanonical activated PAR3-mediated Tie2 activation
Next, blocking antibodies were used to provide insight into the role of Tie2 on FXa-mediated barrier function. Blocking antibodies against Tie2 reduced FXa-mediated barrier protective effects by ∼34%, whereas inhibition of Tie2 did not affect thrombin-mediated barrier disruption (Figure 7A). To understand how PAR3-dependent Tie2 activation contributes to barrier protective effects, changes in the expression of the tight-junction-associated protein zona occludens 1 (ZO-1) were analyzed. FXa and the P3R peptide induced time-dependent increases in ZO-1 expression in EA.hy926 cells (Figure 7B). Maximal ZO-1 upregulation by FXa was achieved after ∼60 minutes and was sustained for up to 3 hours (Figure 7C). The upregulation of ZO-1 induced by the P3R peptide after 3 hours was similar to that induced by FXa (Figure 7D). Blocking antibodies against Tie2 or PAR3 confirmed that the FXa-induced ZO-1 expression required both these receptors (Figure 7E). Finally, immunohistochemistry indicated that the upregulation of ZO-1 induced by FXa and P3R was associated with the relocation of ZO-1 to cell-cell borders and with the formation of a typical ZO-1 honeycomb pattern that is characteristic for the stabilization of tight junctions (Figure 7F).30-34 Thus, phosphorylation of Tie2 induced by FXa and the P3R peptide resulted in functional vascular barrier protective effects that involved ZO-1-mediated stabilization of tight junctions between cells.
Contributions of PAR3 and Tie2 to FXa-induced endothelial barrier protection. (A) Protection of endothelial barrier function by FXa (25 nM; 3 hours) in the presence of blocking antibodies against Tie2 (anti-hTie2; 25 µg/mL). The maximal permeability induced by thrombin in nontreated cells was considered as 100%. (B) Upregulation of ZO-1 compared with β-actin in time after treatment of EA.hy926 cells with FXa (50 nM) or P3R (50 µM). (C) Quantification of the time-dependent ZO-1 upregulation by FXa (50 nM). (D) Quantification of P3R (50 µM) induced ZO-1 upregulation after 3 hours. (E) Effect of blocking Tie2 (anti-hTie2; 25 µg/mL) and PAR3 (Moab19b; 25 µg/mL) antibodies on FXa-induced (50 nM; 3 hours) ZO-1 upregulation. (F) Immunofluorescent staining for ZO-1 and 4,6-diamidino-2-phenylindole (DAPI) in EA.hy926 cells incubated for 3 hours in the absence or presence of FXa (50 nM) or P3R (50 µM). Images were acquired with a fluorescence microscope system (Axio Carl Zeiss Imager.M1) with a ×40/0.75 EC Plan-NEOFLUAR objective and a Zeiss Axiocam MRm black-and-white camera and AxioVision 4.8 (Zeiss) acquisition software (exposure time: DAPI, 2 milliseconds; Alexa488, 200 milliseconds). Images were processed in Adobe Photoshop CS6, image size was adjusted to 300 dpi, contrast and brightness were increased to 100 and 80, respectively, and images were pseudo-colored by compiling the individual grayscale images into the corresponding red-green-blue layers. (A, C, and D) Data points represent the mean ± SD of at least 3 individual experiments. (B,E-F) Shown are typical images of representative experiments. *P < .05. ns, not significant.
Contributions of PAR3 and Tie2 to FXa-induced endothelial barrier protection. (A) Protection of endothelial barrier function by FXa (25 nM; 3 hours) in the presence of blocking antibodies against Tie2 (anti-hTie2; 25 µg/mL). The maximal permeability induced by thrombin in nontreated cells was considered as 100%. (B) Upregulation of ZO-1 compared with β-actin in time after treatment of EA.hy926 cells with FXa (50 nM) or P3R (50 µM). (C) Quantification of the time-dependent ZO-1 upregulation by FXa (50 nM). (D) Quantification of P3R (50 µM) induced ZO-1 upregulation after 3 hours. (E) Effect of blocking Tie2 (anti-hTie2; 25 µg/mL) and PAR3 (Moab19b; 25 µg/mL) antibodies on FXa-induced (50 nM; 3 hours) ZO-1 upregulation. (F) Immunofluorescent staining for ZO-1 and 4,6-diamidino-2-phenylindole (DAPI) in EA.hy926 cells incubated for 3 hours in the absence or presence of FXa (50 nM) or P3R (50 µM). Images were acquired with a fluorescence microscope system (Axio Carl Zeiss Imager.M1) with a ×40/0.75 EC Plan-NEOFLUAR objective and a Zeiss Axiocam MRm black-and-white camera and AxioVision 4.8 (Zeiss) acquisition software (exposure time: DAPI, 2 milliseconds; Alexa488, 200 milliseconds). Images were processed in Adobe Photoshop CS6, image size was adjusted to 300 dpi, contrast and brightness were increased to 100 and 80, respectively, and images were pseudo-colored by compiling the individual grayscale images into the corresponding red-green-blue layers. (A, C, and D) Data points represent the mean ± SD of at least 3 individual experiments. (B,E-F) Shown are typical images of representative experiments. *P < .05. ns, not significant.
Discussion
Here, we identified a novel pathway for Tie2 activation by noncanonical PAR3 activation that promoted tight-junction formation and endothelial barrier protective effects. In contrast, canonical activation of PAR3 enhanced PAR1-mediated barrier disruptive effects by thrombin. These results exemplify the novel dimensions provided by noncanonical activation of PAR3 for the functional selectivity of protease signaling.
FXa activated PAR3 at noncanonical Arg41 and not at canonical Lys38 like thrombin, whereas FXa cleaved PAR1 like thrombin at canonical Arg41 but not at the Arg46 APC cleavage site. This PAR1 and PAR3 cleavage profile of FXa is thus far unique among coagulation proteases and is a crossover between that of thrombin and APC (supplemental Figure 4). In addition to APC, FXa is now the second coagulation protease to activate PAR3 at noncanonical Arg41; thus, noncanonical PAR3 activation is not an aberrant property of APC. The unique PAR1-PAR3 cleavage pattern of FXa provided an opportunity to dissect the contributions of noncanonical PAR3 cleavage vs canonical PAR1-mediated signaling to functional cellular effects. For instance, FXa induced immediate endothelial barrier disruption dependent on activation of PAR1 at Arg41. FXa also provided time-dependent endothelial barrier protective effects that required PAR1 and PAR2 and, in part, were derived from EPCR-dependent noncanonical PAR3 activation resulting in prolonged Tie2 activation, thus illustrating the duality of FXa-mediated cellular effects (supplemental Figure 4).
The selectivity of the noncanonical PAR3 tethered-ligand peptide for Tie2 activation was remarkable because none of the other PAR1, PAR2, or canonical PAR3 tethered-ligand peptides induced Tie2 activation similar to the P3R peptide, but how does noncanonical cleaved PAR3 induce activation of the Tie2 receptor? Typically, activation of the endothelial Tie2 receptor is regulated by a dynamic balance between dummy ligand Ang2 that keeps the receptor quiescent and agonist ligand Ang1.32,35 On endothelial cells, APC enhances Tie2 expression and shifts the Ang1/Ang2 balance by stimulating Ang1 while inhibiting Ang2 expression in an EPCR- and PAR1-dependent manner.10,28 PAR1 activation by thrombin results in opposite effects that increase Ang2 and decrease Ang1, thereby attenuating the Tie2 signaling pathway in endothelial cells.10,36 However, changes in Ang1 and Ang2 are typically determined after 24 hours, whereas P3R-mediated Tie2 activation occurred within 30 to 180 minutes. Thus, our data suggest that mechanisms other than changes in the Ang1/Ang2 ratio are involved in PAR3-mediated Tie2 activation, which may require additional receptor complexes such as the epidermal growth factor receptor or sphingosine 1-phosphate receptor.8,9,16,33
To date, there has been no convincing evidence that PAR3 can signal autonomously, but rather PAR3 is suggested to function as a cofactor or coreceptor.13,17,37 PAR1 contributions to PAR3-dependent Tie2 activation suggest the distinct possibility for transactivation of PAR1 by noncanonical activated PAR3 as part of a PAR1-PAR3 heterodimer.13,19 Noncanonical PAR activation and the formation of PAR-PAR heterodimers greatly expand the repertoire of possibilities for protease-mediated PAR signaling (supplemental Figure 4). PAR1-PAR3 heterodimers that consist of canonical activated PAR1 and PAR3 enhanced ERK1/2 phosphorylation. Remarkably, enhanced ERK1/2 phosphorylation by the P3K peptide was not observed when PAR1 signaling was initiated by the high-affinity agonist TFLLRN instead of the natural PAR1 tethered-ligand SFLLRN. Similarly, contributions of PAR2 to FXa-mediated barrier protection were observed only when barrier disruption was initiated by SFLLRN but not by TFLLRN. Although open to alternative explanations, this may suggest that the susceptibility of PAR1 to allosteric modulation by PAR2 or PAR3 is dependent on the bias of the ligand because SFLLRN is considered more neutral biased relative to TFLLRN. Alternatively, the notion that PAR3 is a nonsignaling receptor is predominantly based on inability of the canonical tethered-ligand peptide to induce signaling or platelet activation. The new insights in biased signaling and noncanonical PAR activation raise the intriguing possibility that PAR3 activation at Arg41 forms a signaling competent tethered ligand that is capable of inducing PAR-mediated signaling, such as inducing the activation of Tie2.
Tie2 is important for the regulation of vascular stability and control of vascular quiescence and was previously implicated to contribute to protection of endothelial and epithelial barrier functions by APC.10,27,28 Activation of Tie2 by noncanonical PAR3 activation resulted in a typical ZO-1 honeycomb pattern that is characteristic for the stabilization of tight junctions.30-34 ZO-1 is a multidomain scaffolding protein that links the transmembrane domains of tight-junction proteins to components of the cortical cytoskeleton. Notwithstanding the potential for integration with other barrier protective signaling pathways, it is clear that the molecular mechanisms for regulation of barrier function by noncanonical PAR3-Tie2 activation differ from those induced by noncanonical PAR1 activation at Arg46 that employ Akt and Rac1 activation or canonical PAR1-PAR2 activation that causes transactivation. Thus, specific cellular effects can be traced back to activation of specific cell-signaling pathways resulting from individual canonical or noncanonical PAR activation, such as the activation of Tie2 attributable to noncanonical PAR3 activation that is shared between APC and FXa. Inversely, the realization that individual canonical or noncanonical PAR activation events are linked to specific cellular effects provides a framework to begin to understand how and why different proteases display functional selectivity with sometimes overlapping and sometimes distinct cellular effects.1,38-42
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr L. Brass (University of Pennsylvania, Philadelphia, PA) for the gift of PAR1 (ATAP2 and WEDE15) and PAR3 (19b) antibodies, Dr Kenji Fukudome (Saga Medical School, Saga, Japan) for the anti-EPCR antibody (rcr-252), and Dr C. J. S. Edgell (University of North Carolina, Chapel Hill, NC) for the EA.hy926 endothelial cells.
This work was supported by a grant from the National Institutes of Health, National Heart, Lung, and Blood Institute (HL104165) (L.O.M.).
Authorship
Contribution: F.S. performed the experiments; and F.S. and L.O.M. designed the research and concept, analyzed results, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Laurent O. Mosnier, Department of Molecular and Experimental Medicine (MEM-180), The Scripps Research Institute, 10550 North Torrey Pines Rd, La Jolla, CA 92037; e-mail: lmosnier@scripps.edu.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal