In this issue of Blood, Lerman et al1 identify the α3β1 integrin as a novel marker for hyperactivated neutrophils in septic patients and septic mice and provide functional evidence that this integrin contributes significantly to neutrophil infiltration and hyperresponsiveness in sepsis. Thus, α3β1 integrin could represent a novel and specific therapeutic target for sepsis.
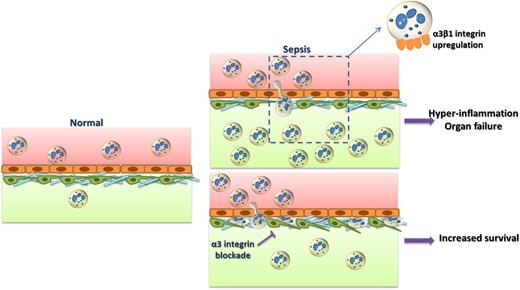
In sepsis, α3β1-integrin expression is upregulated on hyperinflammatory neutrophils and promotes their recruitment to affected sites through interactions with extracellular matrix proteins, such as laminin. Neutrophil infiltration may result in hyperinflammation, tissue damage, and organ failure. Inhibition of α3β1 integrin blocks neutrophil extravasation; neutrophils are entrapped between the endothelium and the pericytes. Inhibition of α3β1 integrin could thereby prevent hyperinflammation and promote survival in sepsis.
In sepsis, α3β1-integrin expression is upregulated on hyperinflammatory neutrophils and promotes their recruitment to affected sites through interactions with extracellular matrix proteins, such as laminin. Neutrophil infiltration may result in hyperinflammation, tissue damage, and organ failure. Inhibition of α3β1 integrin blocks neutrophil extravasation; neutrophils are entrapped between the endothelium and the pericytes. Inhibition of α3β1 integrin could thereby prevent hyperinflammation and promote survival in sepsis.
Despite the advances in the understanding of the mechanisms that contribute to development of systemic inflammation during microbial infection, sepsis is still a major clinical problem with significant mortality, ranging from 15% to 30% in the United States, mainly from the lack of specific treatments.2 Identification of effective therapies is hampered by the heterogeneity and the biphasic nature of sepsis; the initial hyperinflammatory phase is followed by a phase of immune exhaustion when anti-inflammatory therapies are ineffective or even have detrimental effects on survival.3,4 Neutrophils are the first line of defense against invading pathogens; they are recruited in vast numbers to the site of infection, constituting a key cell population in the initial pro-inflammatory phase of sepsis. However, an overwhelming neutrophil response often has detrimental effects that lead to microvasculature injury and contribute to organ damage and failure.4,5 Thus, the inflammatory response, including neutrophil recruitment, in sepsis is a double-edged sword. Although inflammation is needed for pathogen clearance, it also mediates organ damage.6,7 Traditionally, β2 integrins were considered critical players in neutrophil–endothelial interactions and transendothelial migration during inflammation, infection, and sepsis.8,9 However, the constitutive expression of β2 integrins in leukocytes and the impaired pathogen clearance associated with β2-integrin inhibition, which has an adverse effect in survival, renders their blockade in the setting of microbial infection difficult.10
Thus, the need for novel, specific biomarkers to diagnose and stratify septic patients is imperative. In addition, it is important to identify and therapeutically target pathways that specifically mediate neutrophil recruitment and activation in sepsis without having an impact on pathogen clearance.
Lerman et al identified α3β1 integrin as such a therapeutic target. They demonstrated that it is specifically overexpressed in neutrophils in sepsis and plays a role in the hyperresponsiveness of these cells.1 The expression of this integrin on neutrophils is highly upregulated in septic patients but not in patients with noninfectious inflammatory response syndrome; moreover, α3β1-integrin expression was increased in neutrophils from mice subjected to experimental endotoxemia or cecal ligation and puncture-induced sepsis.1 These data suggest that neutrophil α3β1-integrin expression could be a specific biomarker for sepsis. The increased α3β1 expression in a subpopulation of neutrophils correlated with a hyperactivated phenotype and associated with increased interleukin-6 production and myeloperoxidase activity. Strikingly, pharmacologic blockade of α3β1 integrin with the cyclic peptide LXY2 or neutrophil-specific inactivation of α3 not only abrogated neutrophil infiltration into the organs (eg, the lung) of septic mice, but also improved survival to septic shock (see figure) without affecting bacterial clearance.1 The decreased neutrophil extravasation resulting from α3β1-integrin inhibition was associated with neutrophils entrapped in the space between the endothelial cells and pericytes, as shown by intravital and electron microscopy (see figure). These data make a strong case that the interaction between α3β1 integrin and its ligands in the extracellular matrix (eg, laminin) is critical for neutrophil migration beneath the endothelium and through the vascular wall. Additionally, α3β1 integrin was involved in neutrophil activation and interleukin-6 production following TLR2 ligation.1
Taken together, the study by Lerman demonstrates a previously unrecognized role for α3β1 integrin in neutrophil infiltration and activation in response to pathogen-derived stimuli in the course of sepsis. The specific upregulation of α3β1 integrin in septic neutrophils may provide an important novel biomarker and diagnostic tool in sepsis, which merits further investigation in clinical settings. However, the implications of this study are over and above the diagnostic potential of neutrophil α3β1-integrin expression. In particular, α3β1 blockade could represent an attractive novel therapeutic strategy to ameliorate systemic inflammation-induced organ damage and thereby improve survival in sepsis without having a negative impact on pathogen clearance. It is definitively worth planning further preclinical and clinical studies to assess targeting α3β1 integrin in the treatment of sepsis.
Conflict-of-interest disclosure: The authors declare no competing financial interest.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal