Key Points
Rituximab plus liposomal doxorubicin is active and tolerated in patients with symptomatic KSHV-associated multicentric Castleman disease.
This is a safe and effective initial regimen for concurrent symptomatic KSHV-associated multicentric Castleman disease and Kaposi sarcoma.
Abstract
Kaposi sarcoma (KS) herpesvirus–associated multicentric Castleman disease (KSHV-MCD) is a lymphoproliferative disorder, most commonly seen in HIV-infected patients, that has a high mortality if untreated. Concurrent KS is common. Although rituximab has reported activity in KSHV-MCD, its use is often associated with KS progression. Within a natural history study of KSHV-MCD, we prospectively evaluated rituximab 375 mg/m2 combined with liposomal doxorubicin 20 mg/m2 (R-Dox) every 3 weeks in 17 patients. Patients received a median of 4 cycles (range 3-9). All received antiretroviral therapy, 11 received consolidation interferon-α, and 6 received consolidation high-dose zidovudine with valganciclovir. Using NCI KSHV-MCD response criteria, major clinical and biochemical responses were attained in 94% and 88% of patients, respectively. With a median 58 months’ potential follow-up, 3-year event-free survival was 69% and 3-year overall survival was 81%. During R-Dox therapy, cutaneous KS developed in 1 patient, whereas 5 of 6 patients with it had clinical improvement. R-Dox was associated with significant improvement in anemia and hypoalbuminemia. KSHV viral load, KSHV viral interleukin-6, C-reactive protein, human interleukin-6, and serum immunoglobulin free light chains decreased with therapy. R-Dox is effective in symptomatic KSHV-MCD and may be useful in patients with concurrent KS. This trial was registered at www.clinicaltrials.gov as #NCT00092222.
Introduction
Kaposi sarcoma herpesvirus (KSHV), also called human herpesvirus-8, is the etiologic agent of a plasmablastic form of multicentric Castleman disease (KSHV-MCD), a B-cell lymphoproliferative disorder most common in HIV-infected persons.1 KSHV-MCD is characterized by inflammatory symptoms, progressive fatigue, and weight loss. Symptomatic patients generally have elevated C-reactive protein (CRP) and KSHV viral load, and they often have anemia, thrombocytopenia, hypoalbuminemia, hyponatremia, and elevated γ-globulin.2-9 Computerized tomography (CT) typically demonstrates diffuse adenopathy and splenomegaly. Diagnosis requires pathologic confirmation.
KSHV-infected plasmablasts often express KSHV-encoded viral interleukin-6 (vIL-6) and other lytic genes. KSHV is also the etiologic agent of Kaposi sarcoma (KS)10 and primary effusion lymphoma (PEL).11,12 Patients with KSHV-MCD commonly have concurrent KS, often involving lymph nodes, and are at risk of developing KS, PEL, and large-cell lymphoma arising in KSHV-MCD.13-16
KSHV-MCD often has a waxing and waning course. Untreated, it is usually lethal within 2 to 3 years.1,7 Patients can develop severe sepsislike manifestations associated with elevated human IL-6, vIL-6, and IL-10,17 and/or hemophagocytic syndrome.18,19 Such disease manifestations require urgent treatment. In addition, managing patients with more than one KSHV-associated malignancy requires accurate diagnoses and personalized treatment.
Unlike idiopathic MCD,20-23 there is no FDA-approved therapy for KSHV-MCD. Disease control has been reported with chemotherapeutic agents used for lymphoid malignancies,24 liposomal doxorubicin alone,25 interferon-α,6,26,27 thalidomide,28 ganciclovir,29 and splenectomy. Steroids may temporarily reduce inflammation but commonly exacerbate KS and increase infection risk.30 Concurrent combination antiretroviral therapy (HAART) is generally used in HIV-infected patients.4,31
Treatment with virus-activated cytotoxic therapy using high-dose zidovudine combined with valganciclovir (AZT/VGC),2 and rituximab,32-35 a humanized monoclonal antibody against CD20, have each been evaluated prospectively. In 14 patients, AZT/VGC yielded a clinical response rate of 86% using National Cancer Institute (NCI) KSHV-MCD Response Criteria.2 KSHV viral load, vIL-6, IL-6, IL-10, and CRP all decreased significantly. However, median progression-free survival was 6 months, and some patients required subsequent therapy.2
Rituximab was evaluated in two phase 2 studies. In the CastlemaB Study, 24 HIV-infected patients with chemotherapy-dependent KSHV-MCD received rituximab 375 mg/m2 weekly for 4 weeks.34 Ninety-two percent had sustained resolution of MCD symptoms 60 days after initiation of rituximab. At 1 year, 71% were alive and disease free.34 In a second study, 21 patients with symptomatic KSHV-MCD were treated with rituximab 375 mg/m2 weekly for 4 weeks.35 Ninety-five percent had resolution of symptoms and significant decreases in KSHV viral load and CRP. Seventy-nine percent were relapse free at 2 years.35 However, in these rituximab studies, worsening KS was observed in 35% to 67% of patients with baseline KS,34,35 suggesting that additional approaches are required for the substantial subset of KSHV-MCD patients with concurrent KS. The pathophysiology of KS progression in the setting of rituximab is poorly understood but may be caused in part by the effects on KSHV-specific humoral immunity.36-40 Also, rituximab alone may sometimes be inadequate in severe KSHV-MCD.41-43 We combined rituximab with liposomal doxorubicin (R-Dox) with the rationale that liposomal doxorubicin would directly target CD20– KSHV-infected MCD plasmablasts and KS spindle cells, including those in lymph nodes that may provide paracrine stimulation for KSHV-MCD plasmablasts.44 We describe a pilot study of R-Dox in HIV-infected KSHV-MCD patients with concurrent KS and/or inflammatory symptoms of at least moderate severity.
Patients and methods
Eligibility
Starting in 2004, patients with pathologically confirmed KSHV-MCD were enrolled in a natural history protocol (#NCT00099073) with embedded prospective evaluations of several treatments. Patients were eligible for the study of R-Dox followed by consolidation therapy if they had at least 1 symptom and 1 laboratory abnormality attributed to KSHV-MCD. According to the protocol, R-Dox was preferred for patients with symptomatic KSHV-MCD and concurrent symptomatic KS, progression through virus-activated cytotoxic therapy, or disease severe enough to warrant immunochemotherapy (ie, Eastern Cooperative Oncology Group [ECOG] performance status >grade 2 and/or at least 1 symptom or laboratory abnormality that would be considered ≥Grade 3 by NCI Common Terminology for Adverse Advents, version 3.0 [CTCAE v3.0]).8 The protocol was approved by the NCI Institutional Review Board. All patients gave written informed consent in accordance with the Declaration of Helsinki.
Treatment
The goal of therapy was resolution of clinical and biochemical abnormalities attributed to KSHV-MCD with long-term disease remission. Rituximab 375 mg/m2 combined with liposomal doxorubicin (Doxil) 20 mg/m2 was administered intravenously every 3 weeks. Premedications included diphenhydramine 50 mg and acetaminophen 650 mg. A single dose of steroid premedication (dexamethasone 8 mg) was allowed. Treatment was to be guided by KSHV-MCD response; patients received up to 2 cycles beyond resolution of symptoms attributed to KSHV-MCD and marked improvement in biochemical abnormalities. All patients were prescribed HAART. Protocol therapy included consolidation after R-Dox, with interferon-α starting at 7.5 million units subcutaneous injection 3 times weekly and escalating to up to 45 million units 3 times weekly as tolerated. Starting June 2010, AZT 600 mg orally every 6 hours combined with VGC 900 mg orally every 12 hours for 7 days of a 21-day cycle2 were added as an alternative consolidation therapy given the observed adverse event (AE) profile of interferon-α and emerging data on treatment of KSHV-MCD with this therapy. The planned duration of consolidation was 6 to 12 months and was guided by patient ability to tolerate and adhere to therapy. In patients with KS requiring additional therapy, standard-of-care KS therapy was administered and KSHV-MCD consolidation therapy was deferred.
KSHV viral load and serum viral IL-6
KSHV-viral copies per million peripheral blood mononuclear cells (PBMCs) were measured using polymerase chain reaction (PCR).17,45,46 KSHV DNA was assessed in DNA extracted from PBMCs using K6 primers.45 The number of cellular equivalents was determined using a human endogenous retrovirus 3 PCR assay.47 Serum vIL-6 was measured using a sandwich enzyme-linked immunosorbent assay3,17 that does not crossreact with human IL-6 at concentrations up to 10 000 pg/mL.3,17 The lower limit of detection of vIL-6 was 1560 pg/mL.
Other assays
Serum IL-6 and IL-10 and other cytokines were evaluated using the MSD 96-Well Multiarray Proinflammatory 7-plex Assay (Meso-Scale Discovery, Gaithersburg, MD) and the Sector Imager. This assay does not crossreact with vIL-6 at concentrations up to 20 000 pg/mL.17 CD4+ and CD19+ cells were measured by fluorescence-activated cell sorting. Plasma HIV-1 mRNA was measured using Roche Amplicor HIV-1 Monitoring Kits (Roche Diagnostic Systems, Branchburg, NJ). From the study inception until May 2009, a sensitive CRP (sCRP) assay was performed using the Immage Platform (Beckman Coulter, Brea, CA). Subsequently, a high-sensitivity (hsCRP) assay using Siemens Dimension Vista platform (Siemens AG, Munich, Germany) was used, with values corrected using a previously described formula.2 Serum immunoglobulins were evaluated on a Siemens Vista platform, except IgE, which was evaluated on an Immage Platform (Beckman Coulter) from 2006 through 2008. Serum free light chains (FLCs) were measured by the Siemens BNII.
Response evaluations
Patients were monitored on day 1 of each cycle for clinical, biochemical, and radiographic responses using protocol-defined NCI KSHV-MCD criteria.2 Symptoms were graded using CTCAE v3.0. Radiographic responses were based on CT performed every other cycle, and separate responses were calculated for lymphadenopathy and splenomegaly as previously described.2 Clinical decisions were based on review of symptoms probably or definitely attributed to KSHV-MCD; overall patient performance status; and clinical laboratory parameters including complete blood counts, blood chemistry values, and CRP. Correlative studies including KSHV viral load, vIL-6, IL-6, and IL-10 did not guide clinical decision making and were not included in response assessments.
Based on our experience evaluating 28 patients receiving either AZT/VGC or R-Dox using the original NCI KSHV-MCD Response Criteria,2 we subsequently developed a modified KSHV-MCD Clinical Benefit Response Criteria (supplemental Table 1) to facilitate assessment of response and treatment decisions based on the overall evaluation of 8 indicator abnormalities (4 symptom groups and 4 laboratory parameters) most closely associated with disease activity. These criteria were evaluated retrospectively.
The KSHV-MCD natural history protocol did not initially specify formal KS assessments using modified ACTG criteria.48,49 Although generally performed, they were not always done at time points related to R-Dox therapy. The protocol was amended in 2010 to specify timing of KS measurements. Where formal KS response assessments were not available at the end of R-Dox, physician-investigator–reported KS status was noted from records of on-study clinical evaluations and substantiated by formal assessment of measurements and photographs that were subsequently obtained during follow-up.
Safety assessments
Safety was monitored each cycle and during posttherapy natural history evaluations. Evaluations included complete blood counts with differential, serum chemistries, urinalysis, CD4 counts, and HIV viral loads. AEs were graded using CTCAEv3.0.
Statistical considerations
Sample size was based on 2-stage Simon optimal design—with α = 0.15 and β = 0.15—aiming to rule out 5% and targeting a 25% clinical response rate. If one or more of the first 8 patients had at least a PR in one parameter (clinical, biochemical, or radiographic), a total of 14 patients were to be treated with R-Dox, with an option to treat more if 2 or more responded. Given the high response rate, 3 additional patients were treated for a total of 17. Event-free survival (EFS), defined as the time from starting R-Dox to the time of KSHV-MCD progressive disease (PD), and overall survival (OS) were calculated using Kaplan-Meier methodology. Use of KS-specific therapy after completion of R-Dox was not a criterion for KSHV-MCD PD in the absence of symptomatic KSHV-MCD. Patient status was monitored until either a patient was taken off protocol (1) or September 1, 2013.
Changes in correlates of KSHV-MCD activity were evaluated by comparing the difference between untransformed and, when appropriate, transformed values at specific time points using the Wilcoxon signed-rank test. For vIL-6, levels were considered detectable or not with changes evaluated using an exact McNemar test. P < .005 was considered significant, whereas .005 < P < .05 indicated a strong trend. All P values were 2 sided. AEs across all cycles possibly, probably, or definitely attributed to therapy were tabulated.
Results
Patient characteristics
Between March 2006 and September 2010, 17 HIV-positive patients with symptomatic KSHV-MCD were administered R-Dox (Table 1). Median CD4 count was 226 cells/μL (range 21-858); 7 patients had <200 cells/μL. All patients were receiving HAART, 14 for >1 year and 3 newly started within 3 months of entry. Twelve patients had a current or past history of KS (Table 1 and supplemental Table 2). The primary indication for selection of R-Dox included KSHV-MCD symptomatic through other therapy (8), concurrent KS requiring therapy (4), and disease severe enough to warrant immunochemotherapy (5).
Baseline characteristics of 17 patients with symptomatic KSHV-associated MCD treated with R-Dox
| Feature . | Result . |
|---|---|
| Age, median (range) | 43 (34-55) |
| Sex | |
| Men | 16 (94) |
| Women | 1 (6) |
| Race/Ethnicity | |
| White | 7 (41) |
| African American | 4 (24) |
| African | 3 (18) |
| Hispanic | 3 (18) |
| Kaposi sarcoma (KS), total, n (%) | 12 (71) |
| History of cutaneous KS only | 2 (12) |
| KS at baseline | 10 (59) |
| Cutaneous only | 2 (12) |
| In MCD-affected lymph node only | 4 (24) |
| Cutaneous and in MCD-affected lymph node | 4 (24) |
| Median CD4, cells/μL (range) | 226 (21-858) |
| CD4 <200 cells/μL | 7 (41) |
| Time since HIV diagnosis, median years (range) | 7 (1-22) |
| HAART | 17* |
| Time on HAART, median months (range) | 25 (0-11 y) |
| Protease inhibitor–based | 10 (59) |
| Non-nucleoside reverse-transcriptase inhibitor based | 6 (35) |
| Integrase stand transfer inhibitor–based† | 1 (6) |
| Median HIV viral load, copies/mL (range) | <50 (<50-7710) |
| Prior KSHV-MCD therapy | |
| Prior therapy‡ | 14 (82) |
| Median number of therapies (range) | 2 (0-5) |
| Prior KS therapy | |
| Prior therapy | 7 (41) |
| Median number of therapies in treated patients (range) | 2 (1-5) |
| Median KSHV viral load, copies/106 PBMCs§ (range) | 22 222 (0-8.7 million) |
| HIV viral load <50 copies/mL | 11 (65) |
| Clinical symptoms§ | |
| Fever/night sweats | 10 (59) |
| Fatigue | 17 (100) |
| Respiratory symptoms | 9 (53) |
| Gastrointestinal symptoms/anorexia | 11 (65) |
| Biochemical parameters, median (range) | |
| C-reactive protein, mg/dL‖ | 7 (0.4-16.3) |
| Albumin, g/dL | 2.9 (1.2-4) |
| Sodium, mEq/L | 133 (126-138) |
| Platelets, K/µL | 98 (11-567) |
| Hemoglobin, g/dL | 9.3 (6.8-12.8) |
| At least one CTCAE equivalent grade ≥3 symptom/laboratory abnormality or ECOG performance status >2 | 6 (35) |
| Radiographic parameters | |
| Splenomegaly, % (spleen size [cm], range) | 100 (12.5-28) |
| Enlarged lymph nodes, % | 100 |
| Serum immunoglobulins and free light chains‖ | |
| Median IgG, mg/dL (range) | 2680 (1140-5200) |
| Median IgA, mg/dL (range) | 313 (117-1500) |
| Median IgM, mg/dL (range) | 81 (27-167) |
| Median IgE, IU/mL (range) | 240 (70-1940) |
| Median λ free light chains, mg/dL (range) | 7.8 (2-22.6) |
| Median κ free light chains, mg/dL (range) | 6 (1.8-19.8) |
| Feature . | Result . |
|---|---|
| Age, median (range) | 43 (34-55) |
| Sex | |
| Men | 16 (94) |
| Women | 1 (6) |
| Race/Ethnicity | |
| White | 7 (41) |
| African American | 4 (24) |
| African | 3 (18) |
| Hispanic | 3 (18) |
| Kaposi sarcoma (KS), total, n (%) | 12 (71) |
| History of cutaneous KS only | 2 (12) |
| KS at baseline | 10 (59) |
| Cutaneous only | 2 (12) |
| In MCD-affected lymph node only | 4 (24) |
| Cutaneous and in MCD-affected lymph node | 4 (24) |
| Median CD4, cells/μL (range) | 226 (21-858) |
| CD4 <200 cells/μL | 7 (41) |
| Time since HIV diagnosis, median years (range) | 7 (1-22) |
| HAART | 17* |
| Time on HAART, median months (range) | 25 (0-11 y) |
| Protease inhibitor–based | 10 (59) |
| Non-nucleoside reverse-transcriptase inhibitor based | 6 (35) |
| Integrase stand transfer inhibitor–based† | 1 (6) |
| Median HIV viral load, copies/mL (range) | <50 (<50-7710) |
| Prior KSHV-MCD therapy | |
| Prior therapy‡ | 14 (82) |
| Median number of therapies (range) | 2 (0-5) |
| Prior KS therapy | |
| Prior therapy | 7 (41) |
| Median number of therapies in treated patients (range) | 2 (1-5) |
| Median KSHV viral load, copies/106 PBMCs§ (range) | 22 222 (0-8.7 million) |
| HIV viral load <50 copies/mL | 11 (65) |
| Clinical symptoms§ | |
| Fever/night sweats | 10 (59) |
| Fatigue | 17 (100) |
| Respiratory symptoms | 9 (53) |
| Gastrointestinal symptoms/anorexia | 11 (65) |
| Biochemical parameters, median (range) | |
| C-reactive protein, mg/dL‖ | 7 (0.4-16.3) |
| Albumin, g/dL | 2.9 (1.2-4) |
| Sodium, mEq/L | 133 (126-138) |
| Platelets, K/µL | 98 (11-567) |
| Hemoglobin, g/dL | 9.3 (6.8-12.8) |
| At least one CTCAE equivalent grade ≥3 symptom/laboratory abnormality or ECOG performance status >2 | 6 (35) |
| Radiographic parameters | |
| Splenomegaly, % (spleen size [cm], range) | 100 (12.5-28) |
| Enlarged lymph nodes, % | 100 |
| Serum immunoglobulins and free light chains‖ | |
| Median IgG, mg/dL (range) | 2680 (1140-5200) |
| Median IgA, mg/dL (range) | 313 (117-1500) |
| Median IgM, mg/dL (range) | 81 (27-167) |
| Median IgE, IU/mL (range) | 240 (70-1940) |
| Median λ free light chains, mg/dL (range) | 7.8 (2-22.6) |
| Median κ free light chains, mg/dL (range) | 6 (1.8-19.8) |
HAART, combination antiretroviral therapy; CTCAE, Common Terminology Criteria for Adverse Events; ECOG, Eastern Cooperative Oncology Group.
Values are n (%) unless otherwise noted.
Two patients had not taken HAART during the few days before entry because they were too sick to take their oral medications.
Two additional patients received integrase strand transfer inhibitors in combination with either a protease inhibitor or a non-nucleoside reverse-transcriptase inhibitor.
In addition, 8 of these patients also received steroids for KSHV-MCD, and 2 were treated for severe cytopenias before KSHV-MCD diagnosis.
Three patients were receiving steroids and 1 patient was taking valganciclovir at the time of baseline evaluation.
Normal ranges (mg/dL unless noted): c-reactive protein <0.4 mg/dL, IgG 642-1730, IgA 91-499, IgM 34-342, IgE 0-90 (IU/mL), λ free light chains 0.66-2.32, κ free light chains 0.57-2.22.
Most patients had been heavily pretreated for KSHV-MCD and KS. Fourteen patients had prior therapy for KSHV-MCD: cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) (2); cyclophosphamide alone (1); AZT/VGC (9); VGC alone (5); rituximab alone (2); and bortezomib (1). Two were treated for severe autoimmune hemolytic anemia before KSHV-MCD diagnosis with IV immunoglobulin (2), rituximab (1), vincristine (1), and splenectomy (1). Eight patients had received steroids for KSHV-MCD symptom control. In addition, 7 patients had prior KS therapy: liposomal doxorubicin (6), paclitaxel (1), etoposide (1), interleukin-12 (2), thalidomide (2), bevacizumab (1), sorafenib (1), and radiation therapy (1).
Treatment
Patients received a median of 4 cycles of R-Dox (range 3-9). Fifteen received consolidation therapy: 9 were administered interferon-α, (median 10 months, range 2 weeks-10 months) and 6 were administered AZT/ VGC (median 9 cycles, range 6-13). One received liposomal doxorubicin alone for progressive KS after 4 months of consolidation AZT/VGC. One received liposomal doxorubicin alone for 15 cycles after R-Dox to further treat advanced KS.
KSHV-MCD responses
By NCI KSHV-MCD Response Criteria, 15 patients (88%) had a clinical complete response (CR) after R-Dox, and an additional patient had symptom-free disease, yielding 16 patients (94%) with a major clinical response (Table 2). Clinical improvement was generally rapid, with 15 (88%) having a major clinical response after 2 cycles. Two patients previously treated with rituximab both obtained complete clinical and biochemical responses with R-Dox.8 The remaining patient had PD and died with severe inflammatory disease manifestations, worsening KS, and central pontine myelinolysis. PEL was found at autopsy. Thirteen patients (76%) had a biochemical CR and 2 patients had a biochemical partial response (PR), yielding 15 patients (88%) with a major biochemical response. Radiographically, 15 patients (88%) had a major radiographic response. Thirteen (76%) had normalization of lymphadenopathy (CR) and an additional 4 (24%) had a >50% decrease in adenopathy (PR) at the end of R-Dox therapy. Likewise, 8 (47%) had complete resolution of splenomegaly and 7 (41%) had at least a 50% relative decrease in splenomegaly (PR). Overall, 5 (29%) had a CR by all criteria and an additional 9 (53%) had an overall PR, yielding 14 patients (82%) with a major response by all 3 criteria.
Response at end of treatment with rituximab combined with liposomal doxorubicin in 17 patients with symptomatic KSHV-MCD
| NCI-KSHV-MCD Response Criteria . | ||
|---|---|---|
| Response category . | Response . | n(%) . |
| Clinical response | Complete response | 15 (88) |
| Symptom-free disease | 1 (6) | |
| Progressive disease§ | 1 (6) | |
| Major clinical response* | 16 (94) | |
| Biochemical response | Complete response | 13 (76) |
| Partial response | 2 (12) | |
| Major biochemical response† | 15 (88) | |
| Stable disease | 1 (6) | |
| Progressive disease§ | 1 (6) | |
| Radiographic response | Complete response | |
| Nodes | 13 (76) | |
| Spleen | 8 (47) | |
| Partial response | ||
| Nodes | 4 (24) | |
| Spleen | 7 (41) | |
| Major radiographic response‡ | 15 (88) | |
| Stable disease (spleen) | 1 (6) | |
| Progressive disease§ (spleen) | 1 (6) | |
| Overall response | Complete response | 5 (29) |
| Partial response | 9 (53) | |
| Stable disease | 2 (12) | |
| Progressive disease§ | 1 (6) | |
| KSHV-MCD Clinical Benefic Criteria | Complete response | 14 (82) |
| Partial response | 1 (6) | |
| Stable disease | 1 (6) | |
| Progressive disease | 1 (6) | |
| NCI-KSHV-MCD Response Criteria . | ||
|---|---|---|
| Response category . | Response . | n(%) . |
| Clinical response | Complete response | 15 (88) |
| Symptom-free disease | 1 (6) | |
| Progressive disease§ | 1 (6) | |
| Major clinical response* | 16 (94) | |
| Biochemical response | Complete response | 13 (76) |
| Partial response | 2 (12) | |
| Major biochemical response† | 15 (88) | |
| Stable disease | 1 (6) | |
| Progressive disease§ | 1 (6) | |
| Radiographic response | Complete response | |
| Nodes | 13 (76) | |
| Spleen | 8 (47) | |
| Partial response | ||
| Nodes | 4 (24) | |
| Spleen | 7 (41) | |
| Major radiographic response‡ | 15 (88) | |
| Stable disease (spleen) | 1 (6) | |
| Progressive disease§ (spleen) | 1 (6) | |
| Overall response | Complete response | 5 (29) |
| Partial response | 9 (53) | |
| Stable disease | 2 (12) | |
| Progressive disease§ | 1 (6) | |
| KSHV-MCD Clinical Benefic Criteria | Complete response | 14 (82) |
| Partial response | 1 (6) | |
| Stable disease | 1 (6) | |
| Progressive disease | 1 (6) | |
Major clinical response = complete response + symptom-free disease + partial response.
Major biochemical response = complete response + partial response.
Major radiographic response = at least complete response or partial response by both lymph nodes and spleen criteria.
One patient was not evaluable radiographically.
By KSHV-MCD Clinical Benefit Criteria, 14 (82%) patients obtained a CR by the end of R-Dox therapy. One had a PR and one had stable disease (SD); these latter 2 subsequently attained CR during consolidation therapy (94% CR by the end of therapy). One patient had PD.
KS outcomes
Ten patients had baseline KS: 6 had cutaneous KS (of whom 4 also had nodal KS) and 4 had only nodal KS. All nodal KS was in found biopsies that also showed KSHV-MCD. Two additional patients had a history of KS only. KS outcomes are summarized in supplemental Table 2. In the 6 patients with cutaneous KS, after a median of 5 cycles (range 3-7) of R-Dox, KS outcomes compared with baseline were PR (2), SD (1), and documented clinical improvement (3). In the 3 patients with clinical improvement at the end of R-Dox therapy, one had subsequent worsening KS and died; PEL was found at autopsy. The other 2 were subsequently evaluated by formal criteria and obtained a PR or CR. In 1 patient with nodal-only KS at baseline, 20 hyperpigmented skin lesions (2 nodular) developed in cycle 2 that were clinically consistent with KS but not biopsied. These presumptive KS lesions then regressed (PR compared with maximal disease). Thus, only 1 of 17 total patients (6%), or 1 of 10 with baseline cutaneous or adenopathic KS (10%), had KS progression at the end of R-Dox therapy compared with baseline.
During consolidation and follow-up, 1 patient with advanced KS, who had PR after R-Dox, received additional liposomal doxorubicin and had a further PR compared with the end of R-Dox therapy (Figure 1), one who received interferon-α consolidation had a clinical CR, and one who received interferon-α had SD. Only 1 patient had documented KS PD without concurrent worsening of KSHV-MCD, which occurred after 4 months of AZT/VGC consolidation and required therapy. Two others had clinical worsening of KS in the setting of KSHV-MCD; one (described before) had progressive KSHV-MCD in the setting of PEL. The other had worsening KS in the setting of recurrent KSHV-MCD; both KS and KSHV-MCD responded to additional R-Dox therapy. The patient in whom cutaneous KS developed during R-Dox therapy was lost to follow-up.
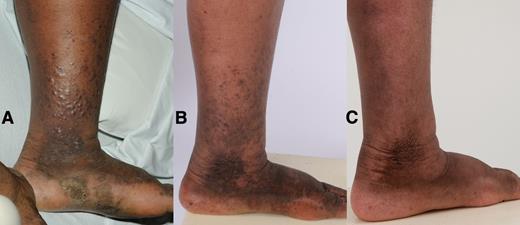
Photograph of representative area of Kaposi sarcoma (KS) in a patient with advanced KS and KSHV-associated multicentric Castleman disease. Photos taken at (A) baseline: >50 lesions on the left lower extremity, many nodular, with tumor-associated edema; (B) after completion of R-Dox: substantial flattening of nodular lesions, decreased tumor-associated edema; and (C) 1 year after completion of additional liposomal doxorubicin: resolution of nodular lesions, some residual pigmentation, and edema.
Photograph of representative area of Kaposi sarcoma (KS) in a patient with advanced KS and KSHV-associated multicentric Castleman disease. Photos taken at (A) baseline: >50 lesions on the left lower extremity, many nodular, with tumor-associated edema; (B) after completion of R-Dox: substantial flattening of nodular lesions, decreased tumor-associated edema; and (C) 1 year after completion of additional liposomal doxorubicin: resolution of nodular lesions, some residual pigmentation, and edema.
Adverse events
Table 3 lists grades 2 through 4 AEs that were at least possibly attributed to each therapy. Overall, R-Dox was well tolerated. The commonest AE was infusion reaction, occurring in 12 (70%) patients during the first dose of rituximab. Patients were generally treated with meperidine and steroids, and rituximab infusion was temporarily delayed. All patients tolerated resumed rituximab. Neutropenia (24% grades 3-4) and anemia (12% grade 3-4) were also common. During consolidation, interferon-α was commonly associated with constitutional symptoms, pain, cytopenia, and proteinuria. Five of 9 (56%) patients had grade 2 depression. In patients receiving AZT/VGC, neutropenia was the commonest AE, with grades 3-4 neutropenia observed in 2 of 5 (40%) patients. Hand-foot syndrome did not develop in any patient.
Important adverse events, worst per patient (% total patients that received therapy): possibly, probably, or definitely attributed to protocol therapies
| Toxicity . | Grade 2 . | Grade 3 . | Grade 4 . |
|---|---|---|---|
| Rituximab combined with liposomal doxorubicin (17 patients, 75 cycles) | |||
| Fever/Infusion reaction | 6 (35) | 3 (18) | — |
| Fatigue | 2 (12) | 1 (6) | — |
| Hematologic | |||
| Anemia | 4 (24) | 1 (6) | 1 (6) |
| Neutropenia | 6 (35) | 3 (18) | 1 (6) |
| CD4 lymphopenia | — | 1 (6) | — |
| Gastrointestinal | |||
| Anorexia | 2 (12) | — | — |
| Gastroesophageal reflux | 1 (6) | — | — |
| Stomatitis | 1 (6) | — | — |
| Pain | |||
| Bone pain | 1 (6) | — | — |
| Headache | 1 (6) | — | — |
| Rash | 1 (6) | — | — |
| Interferon-α (9 patients, 57.5 months) | |||
| Fatigue | 3 (33) | 2 (22) | — |
| Fever | 2 (22) | 2 (22) | — |
| Hematologic | |||
| Anemia | 2 (22) | — | — |
| Thrombocytopenia | 3 (33) | 2 (22) | 1 (11) |
| Neutropenia | 1 (11) | 5 (56) | 3 (33) |
| CD4 lymphopenia | — | 3 (33) | — |
| Gastrointestinal | |||
| Abdominal pain | 1 (11) | — | — |
| Anorexia | 1 (11) | 2 (22) | — |
| Dysgeusia | 1 (11) | — | — |
| Nausea | 1 (11) | — | — |
| Weight loss | 1 (11) | — | — |
| Infection | 1 (11) | — | — |
| Metabolic | |||
| Low albumin | 1 (11) | 1 (11) | — |
| Elevated transaminases | 1 (11) | 3 (33) | — |
| Myalgia | 3 (33) | — | — |
| Neurologic/Psychiatric | |||
| Headache | 1 (11) | — | — |
| Depression | 5 (56) | — | — |
| Insomnia | 1 (11) | — | — |
| Proteinuria | 3 (33) | — | — |
| High-dose zidovudine combined with valganciclovir (5 patients, 46 cycles) | |||
| Fatigue | 1 (20) | — | — |
| Hematologic | |||
| Neutropenia | 1 (20) | 1 (20) | 1 (20) |
| Gastrointestinal | |||
| Abdominal pain | 1 (20) | — | — |
| Anorexia | 1 (20) | — | — |
| Dyspepsia | 1 (20) | — | — |
| Nausea/Vomiting | 2 (40) | — | — |
| Toxicity . | Grade 2 . | Grade 3 . | Grade 4 . |
|---|---|---|---|
| Rituximab combined with liposomal doxorubicin (17 patients, 75 cycles) | |||
| Fever/Infusion reaction | 6 (35) | 3 (18) | — |
| Fatigue | 2 (12) | 1 (6) | — |
| Hematologic | |||
| Anemia | 4 (24) | 1 (6) | 1 (6) |
| Neutropenia | 6 (35) | 3 (18) | 1 (6) |
| CD4 lymphopenia | — | 1 (6) | — |
| Gastrointestinal | |||
| Anorexia | 2 (12) | — | — |
| Gastroesophageal reflux | 1 (6) | — | — |
| Stomatitis | 1 (6) | — | — |
| Pain | |||
| Bone pain | 1 (6) | — | — |
| Headache | 1 (6) | — | — |
| Rash | 1 (6) | — | — |
| Interferon-α (9 patients, 57.5 months) | |||
| Fatigue | 3 (33) | 2 (22) | — |
| Fever | 2 (22) | 2 (22) | — |
| Hematologic | |||
| Anemia | 2 (22) | — | — |
| Thrombocytopenia | 3 (33) | 2 (22) | 1 (11) |
| Neutropenia | 1 (11) | 5 (56) | 3 (33) |
| CD4 lymphopenia | — | 3 (33) | — |
| Gastrointestinal | |||
| Abdominal pain | 1 (11) | — | — |
| Anorexia | 1 (11) | 2 (22) | — |
| Dysgeusia | 1 (11) | — | — |
| Nausea | 1 (11) | — | — |
| Weight loss | 1 (11) | — | — |
| Infection | 1 (11) | — | — |
| Metabolic | |||
| Low albumin | 1 (11) | 1 (11) | — |
| Elevated transaminases | 1 (11) | 3 (33) | — |
| Myalgia | 3 (33) | — | — |
| Neurologic/Psychiatric | |||
| Headache | 1 (11) | — | — |
| Depression | 5 (56) | — | — |
| Insomnia | 1 (11) | — | — |
| Proteinuria | 3 (33) | — | — |
| High-dose zidovudine combined with valganciclovir (5 patients, 46 cycles) | |||
| Fatigue | 1 (20) | — | — |
| Hematologic | |||
| Neutropenia | 1 (20) | 1 (20) | 1 (20) |
| Gastrointestinal | |||
| Abdominal pain | 1 (20) | — | — |
| Anorexia | 1 (20) | — | — |
| Dyspepsia | 1 (20) | — | — |
| Nausea/Vomiting | 2 (40) | — | — |
Values are number (% total patients that received therapy).
— Indicates none observed.
Event-free and overall survival
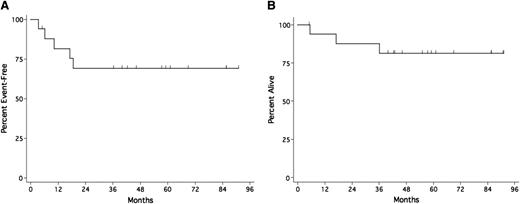
With a median 58-month potential follow-up (range 5-90), including the period of consolidation therapy, EFS was 81.6% at 12 months and 69.0% (95% confidence interval [CI] 44.7%-86.0%) at 24 months and beyond (Figure 2A). One patient had PD and 4 had recurrent symptomatic KSHV-MCD, including one in whom recurrent KSHV-MCD developed during interferon-α consolidation. Three with recurrent disease treated with additional R-Dox all responded. OS was 93.8% at 1 year, 87.5% at 2 years, and 81% (95% CI 57%-93%) at 3 years (Figure 2B). Three patients died: from PEL, pancreatic cancer, and untreated Streptococcus pneumoniae infection in the setting of a possible KSHV-MCD relapse 10 months after the last dose of R-Dox.
Kaplan-Meier survival estimates in patients with symptomatic KSHV-MCD receiving R-Dox. (A) Event-free survival; (B) overall survival.
Kaplan-Meier survival estimates in patients with symptomatic KSHV-MCD receiving R-Dox. (A) Event-free survival; (B) overall survival.
Effect of R-Dox therapy on parameters associated with symptomatic KSHV-MCD, correlative immune biomarkers, and immune reconstitution
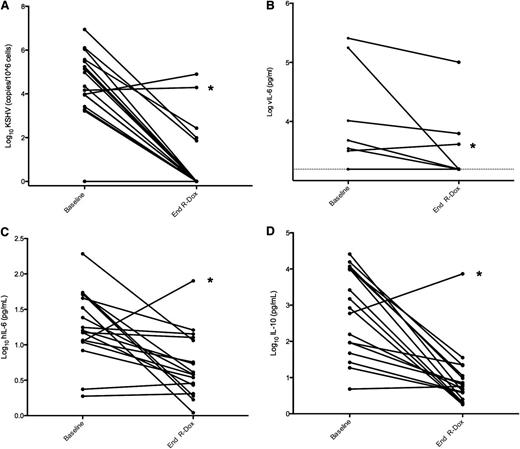
At the end of R-Dox therapy, most parameters associated with symptomatic KSHV-MCD, including KSHV viral load (P = .0002), IL-6 (P = .0026), IL-10 (P = .0002), hemoglobin (<0.0001), albumin (0.0008), and CRP (0.0013), all improved, often substantially (Figure 3 and Table 4). Serum vIL-6 was detectable in 6 patients at baseline and became undetectable in three after R-Dox therapy (Figure 3); given the limited number of subjects with detectable vIL-6, this change was not statistically significant (P = .25). One patient with persistently elevated vIL-6 had PD and was subsequently diagnosed with PEL.
Changes from baseline to end of R-Dox in select biomarkers of KSHV-MCD. (A) Peripheral blood mononuclear cell–associated KSHV viral load (P = .0002, Wilcoxon signed-rank test). (B) Serum viral interleukin-6 (vIL-6) (P = .25, exact McNemar test). (C) Serum human interleukin 6 (hIL-6) (P = .0026, Wilcoxon signed-rank test). (D) Serum human interleukin-10 (IL-10) (P = .0002, Wilcoxon signed-rank test). Log serum vIL-6 was less than the lower limit of detection (1560 pg/mL, or log 3.193) in 11 patients at baseline and 14 patients at the end of R-Dox therapy. *Patient with progressive symptoms and rising KSHV viral load, serum hIL-6, serum IL-10, and serum vIL-6; he subsequently died and was found to have PEL at autopsy.
Changes from baseline to end of R-Dox in select biomarkers of KSHV-MCD. (A) Peripheral blood mononuclear cell–associated KSHV viral load (P = .0002, Wilcoxon signed-rank test). (B) Serum viral interleukin-6 (vIL-6) (P = .25, exact McNemar test). (C) Serum human interleukin 6 (hIL-6) (P = .0026, Wilcoxon signed-rank test). (D) Serum human interleukin-10 (IL-10) (P = .0002, Wilcoxon signed-rank test). Log serum vIL-6 was less than the lower limit of detection (1560 pg/mL, or log 3.193) in 11 patients at baseline and 14 patients at the end of R-Dox therapy. *Patient with progressive symptoms and rising KSHV viral load, serum hIL-6, serum IL-10, and serum vIL-6; he subsequently died and was found to have PEL at autopsy.
Changes in select cytokines, KSHV viral load, clinical labs, and immunology profile in patients with symptomatic KSHV-MCD from baseline to end of therapy with R-Dox
| Laboratory . | Baseline-End R-Dox Median change (range) . | P* . |
|---|---|---|
| Cytokines | ||
| Log10hIL6, pg/mL | −0.46 (−1.49, +0.85) | .0026 |
| Log10IL10†, pg/mL | −1.68 (−3.08, +0.62) | .0002 |
| Log10KSHV, copies/106 PBMCs | −3.96 (−6.04, +0.92) | .0002 |
| Clinical labs used in response criteria | ||
| Hemoglobin, g/dL | +3.9 (−0.3, +10.1) | <.0001 |
| Platelets, ×1000/μL | +78 (−258, +261) | .19 |
| Albumin, g/dL | +0.8 (−1.2, +2.4) | .0008 |
| CRP, mg/dL | −3.0 (−14.7, +2.2) | .0013 |
| Immunologic markers | ||
| κ free light chains, mg/dL | −4.23 (−15.08, +1.69) | <.0001 |
| λ free light chains, mg/dL | −3.53 (−14.78, −0.66) | <.0001 |
| IgG, mg/dL | −390 (−2130, +1160) | .021 |
| IgA, mg/dL | +10, (−200, +174) | .89 |
| IgM, mg/dL | −40 (−98, +102) | .003 |
| IgE, IU/mL | −125 (−1516, +17) | <.0001 |
| CD4 cells/μL | +51 (−359, +345) | .07 |
| CD19 cells/μL | −191 (−81, −873) | <.0001 |
| Laboratory . | Baseline-End R-Dox Median change (range) . | P* . |
|---|---|---|
| Cytokines | ||
| Log10hIL6, pg/mL | −0.46 (−1.49, +0.85) | .0026 |
| Log10IL10†, pg/mL | −1.68 (−3.08, +0.62) | .0002 |
| Log10KSHV, copies/106 PBMCs | −3.96 (−6.04, +0.92) | .0002 |
| Clinical labs used in response criteria | ||
| Hemoglobin, g/dL | +3.9 (−0.3, +10.1) | <.0001 |
| Platelets, ×1000/μL | +78 (−258, +261) | .19 |
| Albumin, g/dL | +0.8 (−1.2, +2.4) | .0008 |
| CRP, mg/dL | −3.0 (−14.7, +2.2) | .0013 |
| Immunologic markers | ||
| κ free light chains, mg/dL | −4.23 (−15.08, +1.69) | <.0001 |
| λ free light chains, mg/dL | −3.53 (−14.78, −0.66) | <.0001 |
| IgG, mg/dL | −390 (−2130, +1160) | .021 |
| IgA, mg/dL | +10, (−200, +174) | .89 |
| IgM, mg/dL | −40 (−98, +102) | .003 |
| IgE, IU/mL | −125 (−1516, +17) | <.0001 |
| CD4 cells/μL | +51 (−359, +345) | .07 |
| CD19 cells/μL | −191 (−81, −873) | <.0001 |
This analysis includes 1 patient who progressed while on R-Dox and was subsequently diagnosed with primary effusion lymphoma.
The Wilcoxon signed-rank test was used to test the difference between the transformed or untransformed values at the identified time points. P < .005 indicates significance, whereas .005 < P < .05 indicates a strong trend.
Untransformed numbers capped at 2500 pg/mL, the upper limit for which the assay is validated.
In addition, we evaluated changes in B-cell parameters, including immunoglobulins and CD19+ B cells. Both HIV and KSHV-MCD are associated with hypergammaglobinemia.6,50 Immunoglobulin FLCs are a useful marker of B-cell lymphoproliferation51,52 and are a predictive factor for lymphoma in HIV infection.52,53 At baseline, 88% had elevated IgG, 94% had elevated IgE (median 240 IU/mL, upper limit of normal [ULN] 90), 94% had elevated κ (median 6 mg/dL, ULN 2.22), and 94% had elevated λ (median 7.8, ULN 2.32) serum FLCs. IgM and IgA were normal in most patients at baseline (Table 1). Compared with baseline, IgE and both κ and λ serum FLCs decreased significantly during R-Dox therapy (P < .0001 for each; Table 4).
Examining CD4+ cells and CD19+ B cells over time (supplemental Figure 1), we found a trend toward an increase in CD4+ cells during R-Dox noted both in patients who had recently started HAART and those on long-standing regimens (median increase 51 cells/μL, P = .07). As expected, CD19+ B cells were markedly decreased (to <10 cells/μL; normal 81-492/μL) at the end of therapy in all patients. Compared with the end of R-Dox therapy, patients had significant CD19+ B-cell immune reconstitution by 6 months (median +88 [range +1, +283]; P < .0001). However, compared with baseline, there was a weak trend toward decreased CD19+ counts persisting 12 months after therapy (median decrease 71 cells/μL; P = .11). In 2 patients, CD19+ lymphocytopenia at 12 months may be partially attributable to additional liposomal doxorubicin given for KS.
We evaluated KSHV-MCD–related clinical parameters 1 year after the end of R-Dox therapy and at least 2 months after consolidation in 13 patients who responded and did not relapse in this period. Compared with baseline, decreases in CRP (median 5 mg/dL; P = .0015), log KSHV viral load (median 4.3 log; P = .0005), and κ (median 3.6 mg/dL; P = .0002) and λ (median 3.1; P = .0002) FLCs were sustained 1 year post R-Dox.
Discussion
This study provides evidence that R-Dox is safe and effective in treating symptomatic KSHV-MCD. Based on the NCI KSHV-MCD Response Criteria, 94% of patients had a major clinical response and 88% had a major biochemical response. Although several criteria have been published, there are no standard criteria for evaluating responses in KSHV-MCD or idiopathic MCD,5,23,34 and harmonization of response criteria would be desirable. The original NCI criteria proved to be somewhat burdensome. We therefore developed a simpler KSHV-MCD Clinical Benefit Response Criteria using 8 classes of clinical abnormalities commonly attributed to KSHV-MCD.5 With these criteria, 88% of patients had a major response (14 CR, 1 PR), and with consolidation, CR was obtained in all but 1 patient. Incorporation of KSHV viral load into response criteria may be worth considering in the future5 ; however current KSHV viral load assays are not FDA-approved for clinical use and vary between groups. Moreover, many centers do not have access to rapid results.
R-Dox therapy may be particularly useful for patients with KSHV-MCD and concurrent KS. New or worsening KS was noted in just 1 patient during R-Dox treatment. Two additional patients had progressive KS after R-Dox, one in the setting of concurrent PEL. Although exact comparisons are difficult, these results are at least as good as or better than previous studies of rituximab alone.8,34,35 In the patient with the most severe KS, 5 cycles of R-Dox led to clinical, biochemical, and radiographic CR of MCD, and a PR of the concurrent KS. With additional liposomal doxorubicin, the patient had near resolution of KS that has been sustained for 4 years with HAART (Figure 1). R-Dox was also effective in patients with severe symptoms or laboratory abnormalities. Of 5 patients with symptoms or laboratory abnormalities CTCAE grade ≥3 and/or an ECOG performance status >2 (but no cutaneous KS requiring therapy), all were symptom free after 1 to 3 cycles and are alive at long-term follow-up. These results suggest that R-Dox may be particularly useful in patients with concurrent KS or relatively severe KSHV-MCD. Rituximab alone can also be effective in KSHV-MCD, and additional studies will be needed to clarify the optimal treatment of different subsets of patients with this disease.
As has been observed with other therapies for KSHV-MCD,2 radiographic outcomes lagged behind clinical outcomes. Although lymphadenopathy and splenomegaly improved in almost every patient, 10 had residual radiographic abnormalities at the end of R-Dox therapy. Of these, one was later diagnosed with PEL. Interestingly, 3 patients with residual splenomegaly (longest dimension 13-19 cm, ULN 12 cm) and 1 asplenic patient with residual lymphadenopathy later had relapse. Of these 4, only one had detectable PBMC-associated KSHV at the end of R-Dox therapy. In contrast, no patient with radiographic CR has relapsed.
Although R-Dox was effective in obtaining clinical remission, recurrent symptomatic KSHV-MCD requiring therapy developed in 4 patients. Improved predictive factors for recurrence are needed. All relapsing patients had residual radiographic abnormalities; indeed, 44% of patients who attained a major clinical response to R-Dox, but with residual radiographic abnormalities, relapsed. This suggests that, as with elevated KSHV-viral load,54 residual lymphadenopathy or splenomegaly may represent inadequate treatment and risk factors for recurrent KSHV-MCD flares. Although most patients received 1 of 2 consolidation regimens, this study was not designed to compare the 2 or assess whether consolidation contributed to KS or KSHV-MCD responses or reduced risk of KSHV-MCD relapse. The roles of consolidation or maintenance therapy as well as optimal management of patients with residual radiographic findings or elevated KSHV viral load remain areas of clinical uncertainty.
Decreases in KSHV viral load, vIL-6, IL-6, and IL-10 were noted with therapy in parallel with clinical improvement, supporting the hypothesis that R-Dox reduces KSHV lytic activation and associated cytokine dysregulation.7,24,55 Interestingly, rituximab is not likely to directly target the KSHV-infected plasmablasts in KSHV-MCD,56,57 because these cells generally do not express CD20.58,59 However, CD20+ cells are noted in KSHV-uninfected lymphocytes within lymph node specimens,16 and rituximab activity likely results in part from its effects on dysregulated CD20+ B cells in the tumor microenvironment.60-65 In addition, liposomal doxorubicin may directly contribute to cytotoxicity in CD20– plasmablasts.
Evaluation of serum immunoglobulin FLCs in patients with symptomatic KSHV-MCD demonstrates both substantial baseline abnormalities and significant effects of therapy. Serum levels of both κ (median 6 mg/dL, 2.7× ULN) and λ (median 7.8, 3.3× ULN) were substantially higher at baseline than those previously described in patients with AIDS but no malignancy, or HIV-infected patients who subsequently developed AIDS-related lymphoma.51,53 Importantly, both κ and λ FLCs decreased substantially with R-Dox and remained significantly lower in asymptomatic patients during follow-up, although modest elevations often persisted. Given the association between elevated FLCs and lymphomagenesis,53 as well as the of high risk of lymphoma noted in patients with KSHV-MCD,13 the effect of rituximab-based therapy on reducing FLCs supports the previous observation of a decreased lymphoma incidence in KSHV-MCD treated with rituximab.14 Control of generalized B-cell lymphoproliferation may be the mechanism by which rituximab reduces lymphomagenesis in this setting. Additional studies of serum FLC in KSHV-associated diseases are warranted.
In conclusion, this study provides evidence that R-Dox is generally well tolerated and has excellent activity in HIV-infected patients with symptomatic KSHV-MCD. It is a particularly important option for patients with concurrent KSHV-MCD and KS. Only 2 patients had CD4+ counts <100 cells/μL, which is a risk factor for infectious morbidity associated with rituximab in patients with HIV-associated systemic lymphomas,66,67 and special precaution may be needed in this patient population. Future studies are required to assess the optimal duration of treatment of KSHV-MCD and the role of consolidation or maintenance therapy. Nonetheless, this study provides additional evidence that with appropriate therapy, durable remissions may be obtained in a majority of HIV-positive patients with KSHV-MCD.
Presented in part at the 51st American Society of Hematology Annual Meeting, San Francisco, CA, December 5, 2009; and as an oral presentation at the 12th International Conference on Malignancies in AIDS and Other Acquired Immunodeficiencies: Basic, Epidemiologic, and Clinical Research, Bethesda, MD, April 26, 2010.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients who volunteered to participate; the medical, nursing, and support staffs of the HIV and AIDS Malignancy Branch; the Medical Oncology Service of the Center for Cancer Research, and the National Institutes of Health (NIH) Clinical Center; Adam Rupert and Randy Stevens and colleagues at Leidos-Frederick, National Cancer Institute (NCI), Frederick, Maryland.
This work was supported in part by the Intramural Research Program, NCI, NIH, and a grant from the NCI, NIH (HHSN261200800001E).
Authorship
Contribution: R.F.L., T.S.U., and R.Y. designed the study; T.S.U., R.F.L., M.N.P., R.Y., K.A., K.M.W., and D.O. cared for patients; T.S.U., M.N.P., S.P., K.A., and K.M.W. collected clinical data; V.M. and D.W. designed and performed KSHV viral-load assays; V.W. helped design and perform the vIL-6 assay; T.S.U., S.M.S., and R.Y. analyzed data; and T.S.U. and R.Y. primarily wrote the manuscript.
Conflict-of-interest disclosure: R.Y.’s spouse is a coinventor on an assay to measure KSHV vIL-6. This invention was made when this scientist was an employee of the United States Government under 45 Code of Federal Regulations Part 7. All rights, title, and interest to this patent have been assigned to the U.S. Department of Health and Human Services. The government conveys a portion of the royalties it receives to its employee-inventors under the Federal Technology Transfer Act of 1986 (P.L. 99-502). The remaining authors declare no competing financial interests.
Correspondence: Thomas S. Uldrick, HIV and AIDS Malignancy Branch, NCI, 10 Center Dr 6N106, Bethesda, MD 20892-1868; e-mail: uldrickts@mail.nih.gov; and Robert Yarchoan, HIV and AIDS Malignancy Branch, NCI, 10 Center Dr 6N106, Bethesda, MD 20892-1868; e-mail: Robert.Yarchoan@nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal