Key Points
Tissue resident group 2 innate lymphoid cells are the main cells producing IL-5 and driving eosinophilia in response to low-dose IL-2 therapy.
We described a novel cellular network activated during IL-2 treatment that may lead to a more efficient use of IL-2 in immunotherapy.
Abstract
Interleukin (IL)-2 promotes regulatory T-cell development and function, and treatment with IL-2 is being tested as therapy for some autoimmune diseases. However, patients receiving IL-2 treatment also experience eosinophilia due to an unknown mechanism. Here, we show that patients receiving low-dose IL-2 have elevated levels of serum IL-5, and this correlates with their degree of eosinophilia. In mice, low-dose IL-2–anti-IL-2 antibody complexes drove group 2 innate lymphoid cells (ILC2) to produce IL-5 and proliferate. Using genetic approaches in mice, we demonstrate that activation of ILC2 was responsible for the eosinophilia observed with IL-2 therapy. These observations reveal a novel cellular network that is activated during IL-2 treatment. A better understanding of the cross talk between these cell populations may lead to more effective targeting of IL-2 to treat autoimmune disease.
Introduction
Treatment with interleukin (IL)-2 has been used for more than 2 decades to enhance antitumor immunity in patients with advanced kidney cancer and melanoma.1,2 Unfortunately, this high-dose IL-2 treatment is associated with side effects (ie, capillary leak syndrome and hepatic and renal dysfunction) limiting its clinical utility.3 IL-5 induced eosinophilia is one of the most common and unwanted effects observed in cancer patients treated with IL-2–based therapy.4 Since the discovery of T-regulatory cells (Treg), studies in mice have shown that low-dose IL-2 therapy actually prevents or ameliorates autoimmune diseases by activating and expanding these cells.5,6 These observations were applied in a first series of studies in humans to treat chronic graft-versus-host disease–related vasculitis and hepatitis C virus (HCV)-related vasculitis.1,7-9 These studies showed that low-dose IL-2 treatment could provide clinical benefits for the patient’s disease with minimal side effects.10 However, in a phase I trial in autoimmune type 1 diabetes (T1D), low-dose IL-2 plus sirolimus (an analog of rapamycin) induced a transient reduction of insulin production, suggesting some residual toxicity, possibly due to toxic effects of the drug on pancreatic β-cells and/or to the activation of non-Treg by IL-2 in this setting.11,12
Study design
Mice and cytokine administration
Red5, YetCre13, and ROSA–diptheria toxin fragment A (DTA) (Gt(Rosa)26DTA) mice were described previously13,14 and injected with IL-2/anti–IL-25 or phosphate-buffered saline (PBS). Mice were maintained in the University of California, San Francisco pathogen-free animal facility in accordance with guidelines established by the Institutional Animal Care and Use Committee and Laboratory Animal Resource Center.
Tissue preparation and flow cytometry
Clinical studies design and participants
Results and discussion
IL-5–induced eosinophilia is one of the most common unwanted side effects observed with high-dose IL-2 immunotherapy.4,16,17 To evaluate if patients treated with low-dose IL-2 also develop eosinophilia, we used data from 2 clinical trials designed to increase Treg cells numbers and induce peripheral tolerance. In the first trial,8 10 individuals with HCV-induced vasculitis received 4 courses of low-dose IL-2 injections that induced a significant increase in serum IL-5 with a variable change in eosinophil counts, which moderately increased over normal values in 12 of 89 evaluations (Figure 1A). However, despite variability and a small number of patients, we observed a strong correlation between increased levels of IL-5 and eosinophils in some patients (Figure 1B). Importantly, there was a significant correlation between eosinophil counts and IL-5 plasma levels in those patients that had detectable IL-5 at baseline (Figure 1B; P = .02). In the second trial,15 T1D patients were treated for 5 days with 3 different doses of IL-2. The cytokine therapy induced a transient and dose-dependent increase in plasma IL-5 levels, with a cumulative effect after each injection of IL-2 (Figure 1C). Overall, these data showed that low-dose IL-2 therapy leads to increased blood concentrations of IL-5 and moderate eosinophilia in some patients. However the mechanism(s) involved in this side effect of the IL-2 therapy was unclear.
IL-2 promotes IL-5–producing ILC2s and induces eosinophilia. (A) HCV-induced vasculitis patients received IL-2 at 1.5 million international units (MIU)/day from days 1 to 5 (course1 [C1]), then at 3 MIU/day from days 15 to 19 (course 2 [C2]), 36 to 40 (course 3 [C3]), and 57 to 61 (course 4 [C4]). IL-5–fold increase (pg/mL) and eosinophil counts in Giga/L were measured just before and after 5 days of IL-2. Normal eosinophil counts in the local laboratory are 0 to 0.7 G/L for men and 0 to 0.5 G/L for women, and are showed as dashed lines. Statistical significance of the differences between the groups was assessed using the Mann-Whitney U test. (B) Correlation between increase in IL-5 and eosinophils for the same patients as in (A). Correlations between eosinophils and IL-5 concentrations were determined by Spearman's correlation coefficient; P = .0218. (C) IL-5–fold increase over the time of T1D patients received a 5-day course of placebo or of IL-2 at the doses of 0.33 MIU/day, 1 MIU/day, and 3 MIU/day. (D) Serum IL-5 concentration in Red5 heterozygous mice treated with phosphate-buffered saline (PBS) or IL-2/anti–IL-2 mAb complex administered every other day for 3 doses. (E) Fluorescence-activated cell sorter plots of IL-5– (Red5 tdtomato reporter) producing cells in the indicated tissues after PBS or IL-2 treatment. (F) Quantitation of CD45+ IL-5+ cells in tissues from IL-5 reporter mice after PBS or IL-2 treatment (VAT: perigonadal VAT). (G) Quantitation of eosinophils (CD45+ CD11b+ SiglecF+) in WT or Rag-deficient (RAG−/−) animals from the indicated tissues after PBS or IL-2/anti–IL-2–treated mice. (H) Fluorescence-activated cell sorter plots of the lineage-defining markers negative subset expressing IL-5 (Red5) in the pancreas after PBS or IL-2 complex (top panel). Characterization of the Red5-producing cells in the pancreas, pre-gated on lineage-negative, CD45+ cells (bottom panels). Mean values ± standard error of the mean. All data were analyzed by comparison of means using unpaired 2-tailed Student t tests. Data are representative of 3 or more experiments or were pooled from 2 to 3 experiments. *P < .05; **P < .01; ***P < .001.
IL-2 promotes IL-5–producing ILC2s and induces eosinophilia. (A) HCV-induced vasculitis patients received IL-2 at 1.5 million international units (MIU)/day from days 1 to 5 (course1 [C1]), then at 3 MIU/day from days 15 to 19 (course 2 [C2]), 36 to 40 (course 3 [C3]), and 57 to 61 (course 4 [C4]). IL-5–fold increase (pg/mL) and eosinophil counts in Giga/L were measured just before and after 5 days of IL-2. Normal eosinophil counts in the local laboratory are 0 to 0.7 G/L for men and 0 to 0.5 G/L for women, and are showed as dashed lines. Statistical significance of the differences between the groups was assessed using the Mann-Whitney U test. (B) Correlation between increase in IL-5 and eosinophils for the same patients as in (A). Correlations between eosinophils and IL-5 concentrations were determined by Spearman's correlation coefficient; P = .0218. (C) IL-5–fold increase over the time of T1D patients received a 5-day course of placebo or of IL-2 at the doses of 0.33 MIU/day, 1 MIU/day, and 3 MIU/day. (D) Serum IL-5 concentration in Red5 heterozygous mice treated with phosphate-buffered saline (PBS) or IL-2/anti–IL-2 mAb complex administered every other day for 3 doses. (E) Fluorescence-activated cell sorter plots of IL-5– (Red5 tdtomato reporter) producing cells in the indicated tissues after PBS or IL-2 treatment. (F) Quantitation of CD45+ IL-5+ cells in tissues from IL-5 reporter mice after PBS or IL-2 treatment (VAT: perigonadal VAT). (G) Quantitation of eosinophils (CD45+ CD11b+ SiglecF+) in WT or Rag-deficient (RAG−/−) animals from the indicated tissues after PBS or IL-2/anti–IL-2–treated mice. (H) Fluorescence-activated cell sorter plots of the lineage-defining markers negative subset expressing IL-5 (Red5) in the pancreas after PBS or IL-2 complex (top panel). Characterization of the Red5-producing cells in the pancreas, pre-gated on lineage-negative, CD45+ cells (bottom panels). Mean values ± standard error of the mean. All data were analyzed by comparison of means using unpaired 2-tailed Student t tests. Data are representative of 3 or more experiments or were pooled from 2 to 3 experiments. *P < .05; **P < .01; ***P < .001.
To determine the mechanism by which IL-2 treatment induced IL-5 and subsequent eosinophilia, we used a newly generated IL-5 reporter mouse.13 As in the human studies, analysis of sera showed an increase in IL-5 production after treatment of mice with low-dose IL-2/monoclonal antibody (mAb) complex (Figure 1D). The IL-5+ cells were mainly present in nonlymphoid tissues such as the lung, visceral adipose tissue (VAT), and pancreas, but not in the spleen, suggesting that the major cells producing IL-5 were not typical circulating lymphocytes (Figure 1E). After IL-2/mAb treatment, IL-5+ cell number strongly increased, with an average fourfold to fivefold increase in cell number (Figure 1E-F). Interestingly, in RAG−/− mice, the numbers of IL-5+ cells in the somatic tissues was equivalent or higher to the numbers seen in wild-type (WT) mice (data not shown). Consistent with the IL-5 data, analysis of eosinophils in IL-2–treated mice showed a pattern with a twofold to threefold increase of eosinophils in both WT and RAG−/− animals (Figure 1G). These results suggest that T- or B-lymphocytes were not necessary for the expansion and/or survival of eosinophils in response to IL-2.
The recent identification of tissue resident group 2 innate lymphoid cells (ILC2) led us to investigate whether these cells were responsible for producing IL-5 in response to IL-2 treatment.18 ILC2 lack the expression of lineage-defining markers, express high levels of Gata3, KLRG1, T1/ST2, and CD25,19,20 and are known to proliferate in response to IL-2.21,22 After IL-2/mAb therapy, we observed that the majority of cells expressing IL-5 have the characteristics of ILC2 (Figure 1H). At steady state, less than 5% of ILC2 were proliferating based on Ki-67 expression, compared with >30% after IL-2/mAb therapy (Figure 2A). This increased proliferation was associated with a twofold to fivefold increased number of ILC2 (Figure 2B) and an increased fluorescence intensity of the IL-5 reporter gene (Figures 1E and 2C). With these conditions, the CD4+ cells constituted only a minor subset (<5%) of the IL-5+ cells (Figure 2C). Interestingly, the eosinophilia induced by the IL-2 therapy was also observed in RAG−/− mice (Figure 1G), whereas the only IL-5–producing cells are ILC2. Under these conditions, we observed an accumulation of tissue ILC2 similar to WT animals treated with IL-2 (Figure 2B). These observations suggested that the eosinophilia generated during this therapy was induced by the activation of ILC2 to produce IL-5.
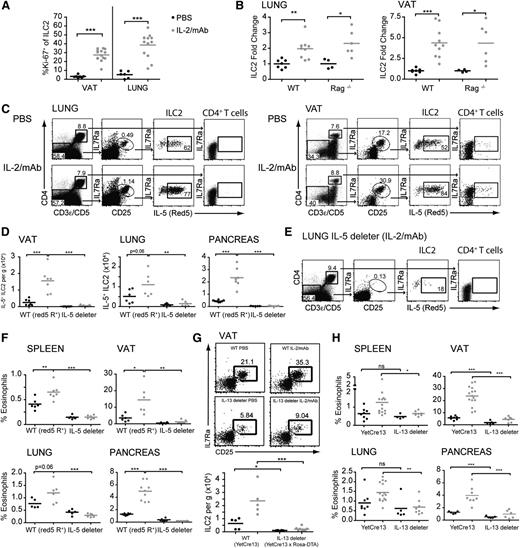
IL-5/13–producing ILC2 induce eosinophilia in response to IL-2 therapy. (A) Quantitation by flow cytometry of ILC2 Ki-67 expression with and without IL-2 treatment. (B) Quantitation of total ILC2 from the indicated strains and tissues, expressed as a fold change from phosphate-buffered saline (PBS)-treated control animals. (C) Fluorescence-activated cell sorter gating of CD4+ T cells and ILC2 (lin− CD127+ CD25+) and expression of IL-5 (Red5) in the strains and tissues indicated after PBS or IL-2 treatment. (D) Quantitation of IL-5+ ILC2 in IL-5 reporter (red5 R+) and IL-5–deleter animals in VAT, lung, and pancreas. (E) Fluorescence-activated cell sorter gating as in (C) for the lung of IL-5–deleter mice treated with IL-2/anti–IL-2 mAb complex. (F) Quantitation of eosinophils in Red5 reporter heterozygous (Red5 R+) or IL-5–deleter animals. (G) Representative fluorescence-activated cell sorter gating (top panels) and quantitation (bottom panel) of VAT ILC2 in control IL-13 reporter animals (YetCre13) or IL-13–deleter (YetCre13 × ROSA-DTA) animals after IL-2 complexes. (H) Quantitation of eosinophils in IL-13 reporter controls (YetCre13) or IL-13–deleter animals. Black line (PBS), gray line (IL-2/mAb). Mean values ± SEM. All data were analyzed by comparison of means using unpaired 2-tailed Student t tests. Data are representative of 3 or more experiments or pooled from 2 to 3 experiments. ns, not significant. *P < .05; **P < .01; ***P < .001.
IL-5/13–producing ILC2 induce eosinophilia in response to IL-2 therapy. (A) Quantitation by flow cytometry of ILC2 Ki-67 expression with and without IL-2 treatment. (B) Quantitation of total ILC2 from the indicated strains and tissues, expressed as a fold change from phosphate-buffered saline (PBS)-treated control animals. (C) Fluorescence-activated cell sorter gating of CD4+ T cells and ILC2 (lin− CD127+ CD25+) and expression of IL-5 (Red5) in the strains and tissues indicated after PBS or IL-2 treatment. (D) Quantitation of IL-5+ ILC2 in IL-5 reporter (red5 R+) and IL-5–deleter animals in VAT, lung, and pancreas. (E) Fluorescence-activated cell sorter gating as in (C) for the lung of IL-5–deleter mice treated with IL-2/anti–IL-2 mAb complex. (F) Quantitation of eosinophils in Red5 reporter heterozygous (Red5 R+) or IL-5–deleter animals. (G) Representative fluorescence-activated cell sorter gating (top panels) and quantitation (bottom panel) of VAT ILC2 in control IL-13 reporter animals (YetCre13) or IL-13–deleter (YetCre13 × ROSA-DTA) animals after IL-2 complexes. (H) Quantitation of eosinophils in IL-13 reporter controls (YetCre13) or IL-13–deleter animals. Black line (PBS), gray line (IL-2/mAb). Mean values ± SEM. All data were analyzed by comparison of means using unpaired 2-tailed Student t tests. Data are representative of 3 or more experiments or pooled from 2 to 3 experiments. ns, not significant. *P < .05; **P < .01; ***P < .001.
To confirm this hypothesis, we used a genetic approach to ablate ILC2. Mice with Cre recombinase engineered under the control of the IL-5 locus were crossed with mice carrying a ROSA-flox-stop-diphtheria toxin, thus ablating all IL-5–producing cells from the mice (termed IL-5–deleter).13,14 Deletion of IL-5+ cells decreased the numbers of ILC2 by > 90% without affecting the total T-cell count (Figure 2D-E and data not shown). More importantly, treatment with IL-2/mAb did not increase the number of eosinophils in the IL-5–deleter mice (Figure 2F). These results suggested that ILC2, which were the major producers of IL-5 in naïve mice, were needed for the eosinophilia induced by IL-2 therapy. However, deletion of IL-5–producing cells also significantly decreased the basal level of the eosinophils; therefore, we decided to use mice with Cre under the control of the IL-13 locus (termed IL-13–deleter). Indeed, fate-mapping techniques have shown that IL-13 is expressed only in a subset of ILC2,14,23 and that IL-13 expression in ILC2 is induced upon activation.13 Under steady-state conditions, most ILC2 expressed low levels of IL-13, leading to a reduction in ILC2 deletion as compared with IL-5–deleter mice (Figure 2D-G). Furthermore, the frequency of eosinophils was not significantly affected in IL-13–deleter mice, except for minor reductions in pancreatic and VAT ILC214 (Figure 2H). During IL-2/mAb treatment, ILC2 expansion was abrogated and the percentage of eosinophils was not significantly increased in the IL-13–deleter mice (Figure 2G-H). These observations confirm the hypothesis that activated ILC2 were the main cell population involved in the development of eosinophilia with IL-2 therapy.
The clinical use of low-dose IL-2 therapy is a promising approach to improve peripheral immune tolerance. However, the early results of IL-2 treatment and clinical outcome has been complex.10 Although clinical improvements have been observed in patients with HCV-induced vasculitis8 and graft-versus-host disease,7,9 T1D patients treated with low-dose IL-2 in conjunction with sirolimus showed a transient β-cell dysfunction, despite an increase in Treg.11 The absence of clinical benefit was associated with an increase in activated non-Tregs (such as natural killer cells and eosinophils) that might have adversely impacted islet function.10 These observations suggest that a better understanding of the targets of IL-2 therapy would be important in designing and evaluating future clinical studies. By using the reporter and deleter mouse model, we provide the first direct evidence that eosinophilia induced by IL-2/anti-IL-2 mAb treatment is due to the activation of tissue resident ILC2 resulting in the release of IL-5. Interestingly, under the same condition, blood ILC2s did not accumulate (data not shown). Since peripheral blood mononuclear cells were the only clinical samples available from patients treated with low-dose IL-2, we monitored serum IL-5 concentration and eosinophils counts as a surrogate readout to evaluate the activation of ILC2. The IL-2 therapy led to increased IL-5 levels in sera and eosinophils numbers among the peripheral blood mononuclear cells, suggesting that in addition to targeting Treg, low-dose IL-2 therapy also induces the activation of ILC2 that release IL-5 and drive eosinophilia. However, to directly assess the role of ILC2 in the immunologic outcome and clinical efficacy of IL-2 immunotherapy in humans, more experiments must be conducted where access to tissues is feasible. In conclusion, these observations reveal a novel cellular network activated during IL-2 treatment and provide new information that may lead to a more efficient use of IL-2 in immunotherapy and contribute to a better understanding of the side effect induced by this cytokine in the clinic.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Michel DuPage for helpful comments on the manuscript, Z.-E. Wang and M. Lee for their technical assistance, and M. Consengco and D. Fuentes for their animal support. This work was supported by grants from the National Institutes of Health, National Institute of Allergy and Infectious Diseases (AI) and National Institute of Diabetes and Digestive and Kidney Diseases (DK) (AI026918, AI030663, AI078869, HL107202, AI046643, AI107328, K08DK101604, AI102011, AI107328, and DK63720), and grants from the University of California, San Francisco Diabetes Family Fund (A.B.M.), the Department of Laboratory Medicine discretionary fund (A.B.M.), the Sandler Asthma Basic Research Center at the University of California, San Francisco, and the Howard Hughes Medical Institute.
Authorship
Contribution: A.B.M. and F.V.G. designed experiments; A.B.M., F.V.G., M.M.M., and M.R. performed research; A.B.M., F.V.G., M.M.M., M.R., D.K., R.M.L., and J.A.B. analyzed data; H.E.L. provided reagent; and A.B.M., F.V.G., D.K., R.M.L., and J.A.B. wrote the manuscript.
Conflict-of-interest disclosure: M.R. and D.K. are inventors of a patent application claiming the use of low-dose IL-2 for treating autoimmune disease, which is owned by their academic institutions and is licensed to ILTOO Pharma in which they hold shares. The remaining authors declare no competing financial interests.
Correspondence: Richard M. Locksley, MS 1032B, Box 0795, 513 Parnassus Ave, San Francisco, CA 94143; e-mail: locksley@medicine.ucsf.edu; and Jeffrey A. Bluestone, S-115 Box 0400, 513 Parnassus Ave, San Francisco, CA 94143; e-mail: jeff.bluestone@ucsf.edu.
References
Author notes
F.V.G. and A.B.M. contributed equally to this study.

![Figure 1. IL-2 promotes IL-5–producing ILC2s and induces eosinophilia. (A) HCV-induced vasculitis patients received IL-2 at 1.5 million international units (MIU)/day from days 1 to 5 (course1 [C1]), then at 3 MIU/day from days 15 to 19 (course 2 [C2]), 36 to 40 (course 3 [C3]), and 57 to 61 (course 4 [C4]). IL-5–fold increase (pg/mL) and eosinophil counts in Giga/L were measured just before and after 5 days of IL-2. Normal eosinophil counts in the local laboratory are 0 to 0.7 G/L for men and 0 to 0.5 G/L for women, and are showed as dashed lines. Statistical significance of the differences between the groups was assessed using the Mann-Whitney U test. (B) Correlation between increase in IL-5 and eosinophils for the same patients as in (A). Correlations between eosinophils and IL-5 concentrations were determined by Spearman's correlation coefficient; P = .0218. (C) IL-5–fold increase over the time of T1D patients received a 5-day course of placebo or of IL-2 at the doses of 0.33 MIU/day, 1 MIU/day, and 3 MIU/day. (D) Serum IL-5 concentration in Red5 heterozygous mice treated with phosphate-buffered saline (PBS) or IL-2/anti–IL-2 mAb complex administered every other day for 3 doses. (E) Fluorescence-activated cell sorter plots of IL-5– (Red5 tdtomato reporter) producing cells in the indicated tissues after PBS or IL-2 treatment. (F) Quantitation of CD45+ IL-5+ cells in tissues from IL-5 reporter mice after PBS or IL-2 treatment (VAT: perigonadal VAT). (G) Quantitation of eosinophils (CD45+ CD11b+ SiglecF+) in WT or Rag-deficient (RAG−/−) animals from the indicated tissues after PBS or IL-2/anti–IL-2–treated mice. (H) Fluorescence-activated cell sorter plots of the lineage-defining markers negative subset expressing IL-5 (Red5) in the pancreas after PBS or IL-2 complex (top panel). Characterization of the Red5-producing cells in the pancreas, pre-gated on lineage-negative, CD45+ cells (bottom panels). Mean values ± standard error of the mean. All data were analyzed by comparison of means using unpaired 2-tailed Student t tests. Data are representative of 3 or more experiments or were pooled from 2 to 3 experiments. *P < .05; **P < .01; ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/24/10.1182_blood-2014-07-587493/4/m_3572f1.jpeg?Expires=1767712701&Signature=pL5rkrPVX5CuoIQFdyZJPg8wxkQHiRKNFJ0mGQz99Dwy0SpTqZa-hoRAxLGuC5mouHZWPUiO1ARJeMoLeqWAQnN2DFBqfzSDUzdeWtS063-UzhXjIycWTfjVSo4WOTnW-VJh9rSOZgfUTUahR91UsJE-DaYc6q70qiS0tSkMrCIRZFzK5u6td9-YNvwA2p3vkEmmBwzk3niP7bLOsEBr9KLmgJMT8HWt0TmQJCCsmW40UIvom2xomzH4umji7SWVKgJT-aoHfEWj8LcdLR8MFJU0MhgOS0IeV4kAswgfs6HxUfyIeWGjBFZw2bPErvwrBk1ppMVWaJKckROhh9AqdQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)