Key Points
Normal maturation of human NK cells requires the expression of TOX2.
TOX2 directly regulates the expression of T-BET during human NK cell development.
Abstract
Thymocyte selection-associated high mobility group box protein family member 2 (TOX2) is a transcription factor belonging to the TOX family that shares a highly conserved high mobility group DNA-binding domain with the other TOX members. Although TOX1 has been shown to be an essential regulator of T-cell and natural killer (NK) cell differentiation in mice, little is known about the roles of the other TOX family members in lymphocyte development, particularly in humans. In this study, we found that TOX2 was preferentially expressed in mature human NK cells (mNK) and was upregulated during in vitro differentiation of NK cells from human umbilical cord blood (UCB)-derived CD34+ cells. Gene silencing of TOX2 intrinsically hindered the transition between early developmental stages of NK cells, whereas overexpression of TOX2 enhanced the development of mNK cells from UCB CD34+ cells. We subsequently found that TOX2 was independent of ETS-1 but could directly upregulate the transcription of TBX21 (encoding T-BET). Overexpression of T-BET rescued the TOX2 knockdown phenotypes. Given the essential function of T-BET in NK cell differentiation, TOX2 therefore plays a crucial role in controlling normal NK cell development by acting upstream of TBX21 transcriptional regulation.
Introduction
Natural killer (NK) cells are lymphocytes of the innate immune system that function to eliminate virally infected cells and malignant cells. NK reconstitution is slow after cord blood transplantation, thus rendering the recipients susceptible to infections and cancer recurrence. Better knowledge in molecular regulation of NK cell development will assist in the discovery of novel agents to hasten NK immune recovery after transplantation. Blood NK cells develop primarily in bone marrow (BM) and then in secondary lymphoid tissues.1 This process is regulated intrinsically through the transcription factor network in NK cells and extrinsically by cytokine-mediated cell-to-cell communication.2-4 The cytokine IL-15, which is produced mainly by phagocytes and dendritic cells, is known to play a pivotal role in NK cell maturation, survival, and homeostasis. Immunodeficient patients with a defect in IL-15 signaling exhibit impaired NK cells.5 Gene deletion of IL-15 or one of its receptor subunits (α/β/γ) greatly impairs NK cell development in mice.6
Studies of various gene knockout mouse models have shown that multiple transcription factors such as E4BP4, ETS1, ID2, T-BET, EOMES, and TOX1 play essential regulatory roles at different stages in NK cell development in mice.7,8 E4BP4 has been shown previously to control early stages of NK cell development.9 Knockout of Nfil3 encoding E4BP4 in mice resulted in a substantial reduction of immature (iNK) and mature NK (mNK) cells in BM. Moreover, the expressions of multiple downstream molecules such as EOMES, ID2, GATA3, and IL-15 receptor β (IL-15RB/CD122) have been shown to be regulated by E4BP4.9,10 ETS1 has also been shown to function at early stages of NK cell development to promote the expression of various transcription factors including T-BET and ID2.11 In mNK cells, the expression of ETS1 is required for the expression of different NK cell receptors such as NKp46, Ly49H, and Ly49D.
Both ID2 and T-BET are involved in the late stages of NK cell differentiation. Deletion of Id2 from mice did not affect the development of NK progenitors (NKPs) and iNK. However, the number of mNK was significantly decreased in periphery.12 Similarly, T-BET–deficient mice have reduced NK cell numbers in the spleen, liver, and peripheral blood with the accumulation of NK cells in lymph nodes and BM.13 Furthermore, T-BET regulates the expression of sphingosine-1 phosphate receptor 5 (S1P5) that plays an important role in NK cell recirculation.14 Although the expression of EOMES is essential for mouse NK cell maturation in the BM,15 a distinct lineage of NK cell population with an immature TRAIL+DX5− phenotype has been identified in the liver that lacks the expression of EOMES. Daussy et al showed that the expression of EOMES was inversely correlated with that of T-BET,16 suggesting a differential control of different tissue NK cell lineages by the interplay between these 2 transcription factors.
TOX1, a founding member of the thymocyte selection-associated high mobility group box protein family, has also been shown to play a role in NK cell maturation. Aliahmad et al showed that Tox1−/− mice had a similar phenotype to Id2−/− mice in which the number of mNK cells in the periphery and lymph nodes was significantly reduced.17 Although the expression of ID2 directly correlated with the level of TOX1, overexpression of ID2 could not rescue the developmental defect of NK cells in Tox1−/− mice. Other transcription factors, such as TCF1, BLIMP1, MEF, and IRF2 are also involved in different stages of NK cell development.7 However, the precise hierarchy of the transcriptional regulation of human NK cell maturation remains unclear.
TOX2 is another member belonging to the TOX family.18 The high mobility group box domain of TOX2 shows a high amino acid homology (>92%) to the other members, whereas the regions outside this domain are less conserved. A previous study of the rat ortholog GCX-1 showed exclusive expression of TOX2 in reproductive tissues, suggesting a specific role in follicular development.19 Although TOX1 has been shown to play key regulatory roles in the development of lymphoid tissue inducer cells, all CD4+ T lineages, and NK cells,17,20,21 the function of TOX2, particularly in human lymphoid development, have not been studied. Here we show that TOX2 is preferentially expressed in human NK cells among various leukocyte populations and is required for in vitro and in vivo human NK cell differentiation from UCB–derived CD34+ hematopoietic stem cells (HSCs). Knockdown of TOX2 reduces the expression of T-BET during NK cell development, hindering NK cell maturation.
Material and methods
Antibodies, vector constructs, and BM cells
The following mAbs were purchased from BD Biosciences (Franklin Lakes, NJ): CD56 (B159, MY31), CD117-APC (YB5.B8), CD3 (SK7), CD34 (8G12), CD107a (H4A3), NKG2D (1D11), and CD16 (3G8). CD94 (131412) mAbs was purchased from R&D Systems (Minneapolis, MN). Goat anti-TOX2 was from Santa Cruz (Dallas, TX), and tubulin (DM1α) mAb was from Sigma (St. Louis, MO). NKG2A (Z199), NKp30 (Z25), NKp44 (Z231), and NKp46 (BAB281) mAbs were obtained from Beckman Coulter (Pasadena, CA).
CIG vector was modified from CL20c (St. Jude Children’s Research Hospital vector laboratory) by inserting a multiple cloning site and an internal ribosome entry site (IRES) upstream of green fluorescent protein (GFP). TOX2 shRNA (TRCN0000021292) or nontargeting control (SHC002) with U6 promoter (Sigma, St. Louis, MO) were amplified and cloned into CIG at the MluI site. Overexpression constructs of TOX2-WT, TOX2-INS (shRNA insensitive TOX2 harboring 5 mutations at the wobble positions), ETS1, E4BP4, ID2, and VDUP1 were amplified from NK cell cDNA and cloned into CIG-Control or CIG-TOX2-KD at the multiple cloning sites upstream of IRES-GFP.
BM cells from healthy donors were purchased from Lonza (Walkersville, MD).
Culture of EL08-1D2 cells
The murine embryonic liver cell line EL08-1D2 was cultured as described previously.22
Isolation of CD34+ cells from UCB
UCB was anonymously obtained from St. Louis Cord Blood Bank (St. Louis, MO). Mononuclear cells were obtained by Ficoll-Hypaque (specific gravity, 1.077; GE Healthcare, Pittsburgh, PA) density gradient centrifugation. CD34+ progenitor cells were isolated using a human CD34 microbead kit (Miltenyi Biotec, Auburn, CA).
Lentivirus transduction of UCB CD34+ cells
CD34+ cells were plated in a 48-well plate in StemSpan SFEM (Stemcell Technologies Inc., Vancouver, BC, Canada) with 1X StemSpan CC100 and 50 ng/mL thrombopoietin for 1 to 2 days. Lentiviral vectors were then transduced in CD34+ cells by spinoculation. GFP+ cells were sorted on fluorescence-activated cell sorter (FACS) Aria (BD Biosciences, Franklin Lakes, NJ) in some experiments as described in the figure legends.
NK cell differentiation in vitro
UCB-derived CD34+ cells (15 000 cells per well) were plated in a 24-well plate on an irradiated confluent monolayer of EL08-1D2 cells in stem cell growth medium (CellGenix, Portsmouth, NH) supplemented with 20% male human AB-sera (Valley Biomedical, Winchester, VA), IL-3 (5 ng/mL), IL-7 (20 ng/mL), IL-15 (10 ng/mL), Flt3L (10 ng/mL), stem cell factor (20 ng/mL), and IL-21 (10 ng/mL) at the start of the culture. Weekly thereafter, 10% human AB-sera and the previously mentioned cytokines except IL-3 were used in subsequent medium changes by demi-depletion (50% volume change).
NK cell differentiation in vivo in humanized mouse model
CD34+ cells transduced with control or TOX2-KD were injected intravenously into the tail vein of 8- to 12-week-old NOD-scid gamma (NSG) (NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ) mice (Jackson Laboratory, Bar Harbor, ME) after irradiation with 250 rad. Six weeks after transplantation, NSG mice were injected intraperitoneally with 2.5 μg of RLI every 5 days for 3 doses. Three days after the last injection, BM cells were harvested for analysis. All mice were used in accordance with the St. Jude Children's Research Hospital-approved animal study.
Quantitative real-time PCR assay and microarray analysis
For RT-q polymerase chain reaction (PCR), total RNA was extracted from developing NK cells at various time points by using RNA Clean & Concentrator (Zymo Research, Irvine, CA). All cDNA was generated by using the SuperScript VILO cDNA Synthesis kit (Life Technologies, Carlsbad, CA) and was diluted 10-fold for analysis using the 7900HT Fast Real-time PCR System (Applied Biosystems, Foster City, CA). The data were calculated as the CT of target genes normalized to the CT of GAPDH for each sample. Primer sequences of the target genes were: TOX2-F 5′-AGTCGGAAGTGCATTTCAAGAT-3′, TOX2-R 5′-GGCCTGAGTGTCTCTGAAGA-3′, IL-15RB-F 5′-ATGGCACTTCCCAGTTC-ACAT-3′, IL-15RB-R 5′-TCACAGGTTTGGTTCCACCG-3′, ETS-1-F 5′-ACGATAGTTGTGATCGCCTCA-3′, ETS-1-R 5′-CCATAGCTGGATTGGTCCACT-3′, ID2-F 5′-GACCCGATGAGCCTGCTATAC-3′, ID2-R 5′-AATAGTGGGATGCGAGTCC-AG-3′, TOX1-F 5′GCACCTGAATGAAGTTGAGTCT-3′, TOX1-R 5′-CCTGGCC-CAGCATATTGGAG-3′, T-BET-F 5′-CAAGGGGGCGTCCAACAAT-3′, T-BET-R 5′- TCTGGCTCTCCGTCGTTCA-3′, NFIL3-F 5′-AAAATGCAGACCGTCAAAA-3′, NFIL3-R 5′-TGACACTTCCGTTAAAGCAG-3′.
Microarray analysis was performed using the GeneChip U133 Plus 2.0 expression microarray (Affymetrix, Santa Clara, CA) on developing NK cells at various time points from 3 donors (GEO accession number GSE58589). Differentially expressed transcripts were analyzed by analysis of variance (ANOVA) using the Spotfire program (Tibco Software, Somerville, MA).
CD107a degranulation assay
K562 cells were incubated with control or TOX2-KD developing cells on day 17 (E:T ratio = 1:2) for 2 hours. CD107a mobilization to the cell surface of the GFP+CD94+CD56+ cell population was analyzed by flow cytometry.
Perforin staining
Cells were fixed with 2% paraformaldehyde at room temperature for 20 minutes and then were permeabilized with 90% methanol on ice for 20 minutes. Intracellular perforin was stained with mouse anti-human perforin-PE (BD Pharmingen, San Diego, CA) and analyzed by flow cytometry.
Promoter reporter assay and TOX2 pull-down
293T cells were cotransfected with 1 μg of various promoter luciferase reporter plasmids and 1 μg of either TOX2-WT or control plasmid. After a 48-hour incubation, 60 million cells of each sample were collected to assay for luciferase activities using Bright-Glo substrate according to the manufacturer’s instructions (Promega, Madison, WI).
Biotinylated T-BET-, IL-15RB-, and ULBP2-promoter probes were generated by PCR using 5′-biotinylated forward primers and the cloned promoter reporter constructs as templates. Total cell lysate was collected from 293T cells 2 days after the transfection with TOX2-WT overexpression construct and then was incubated with streptavidin microbeads (Miltenyi Biotec, Auburn, CA) in the presence or absence of the biotinylated promoter probes for 4hours at 4°C. Proteins pulled down by the microbeads were eluted for western blot analysis for the detection of TOX2.
Results
TOX2 is increasingly expressed during human NK cell development
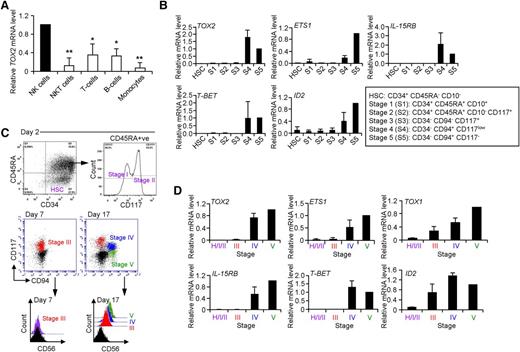
It was shown previously that human CD34+ cells isolated from UCB can differentiate into CD3−CD56+ NK cells in vitro (supplemental Figure 1A, available on the Blood Web site).23 To investigate the changes in gene expression during human NK cell development and to identify novel regulatory pathways, we performed microarray analysis of cultured cells at several time points. We observed a dramatic upregulation of transcription factor TOX2 during in vitro NK cell differentiation, which was confirmed by RT-qPCR (supplemental Figure 1B). Furthermore, TOX2 was predominantly expressed in blood mNK cells but was found at relatively low levels in other types of leukocytes (Figure 1A). We next isolated different developmental stages of NK cells (based on the expression of CD34, CD117, CD94, CD10, and CD45RA as described by Freud et al)24 from BM collected from healthy donors for RT-qPCR analysis (Figure 1B). There was no TOX2 expression in HSCs and there were only trace amounts of TOX2 mRNA detectable during early NK cell development (stage 1 to stage 3). The transcription factor was greatly increased in the late developmental stages (stages 4 and 5) in which its expression profile was similar to that of ETS1, IL-15RB (CD122), and T-BET but different from the early expression pattern of ID2.
TOX2 expression increases during NK cell development. (A) Peripheral blood mononuclear cells collected from healthy donors were subjected to flow-based cell sorting for NK cells (CD3−CD56+), NKT cells (CD3+CD56+), T cells (CD3+CD56−), B cells (CD3−CD19+), and monocytes (CD14+). The expression of TOX2 was analyzed by RT-qPCR (n = 3) *P < .005, **P < .001. (B) Different developmental stages of NK cells were isolated by flow sorting from normal BM samples. The expression of TOX2 and a panel of genes involved in NK cell development were analyzed by RT-qPCR (n = 3). (C) Different developmental stages appeared during in vitro NK cell development from UCB-derived CD34+ cells and were analyzed by flow cytometry at different time points (day 2, day 7, and day 17). Results are representative of 4 independent experiments. (D) Different developmental cell populations were flow sorted at various time points: a mixed population consisting of HSCs, stage I and stage II on day 2, stage III on day 7, and stage IV and stage V on day 17. RNA samples were collected from the sorted cells for cDNA synthesis. RT-qPCR was performed to analyze the expression of TOX2 and a panel of NK cell developmental genes (n = 4).
TOX2 expression increases during NK cell development. (A) Peripheral blood mononuclear cells collected from healthy donors were subjected to flow-based cell sorting for NK cells (CD3−CD56+), NKT cells (CD3+CD56+), T cells (CD3+CD56−), B cells (CD3−CD19+), and monocytes (CD14+). The expression of TOX2 was analyzed by RT-qPCR (n = 3) *P < .005, **P < .001. (B) Different developmental stages of NK cells were isolated by flow sorting from normal BM samples. The expression of TOX2 and a panel of genes involved in NK cell development were analyzed by RT-qPCR (n = 3). (C) Different developmental stages appeared during in vitro NK cell development from UCB-derived CD34+ cells and were analyzed by flow cytometry at different time points (day 2, day 7, and day 17). Results are representative of 4 independent experiments. (D) Different developmental cell populations were flow sorted at various time points: a mixed population consisting of HSCs, stage I and stage II on day 2, stage III on day 7, and stage IV and stage V on day 17. RNA samples were collected from the sorted cells for cDNA synthesis. RT-qPCR was performed to analyze the expression of TOX2 and a panel of NK cell developmental genes (n = 4).
Similar developmental stages can also be identified during in vitro NK cell development from human UCB-derived CD34+ cells.22,24,25 On Day 2 of in vitro cell differentiation, a mixed cell population including HSCs, stage I (CD34+, CD45RA+, CD117−) and stage II (CD34+, CD45RA+, CD117+) cells was observed (Figure 1C). By Day 7, the cells gradually lost the expression of CD34 and continued to express CD117 to enter stage III (CD117+CD94−CD56−). CD56 expression gradually increased while CD117 was gradually decreasing. The cells then started to acquire CD94 to become stage IV (CD117lowCD94+CD56+) and eventually mNK cells (stage V, CD117−CD94+CD56+). In line with the results from BM analysis, similar expression profile of TOX2, ETS1, IL-15RB, T-BET, and ID2 was observed in different developmental stages sorted from the UCB-derived CD34+ cell culture at different time points (day 2 for HSCs, stage I and stage II, day 7 for stage III, and day 17 for stage IV and V) (Figure 1D). We also analyzed TOX1 expression in different developmental stages. Although the 2 transcription factors belong to the same family, the expression profile of TOX1 was different from that of TOX2 in which TOX1 was detectable in earlier developmental stages along with ID2.
TOX2 regulates human NK cell maturation and cytotoxicity
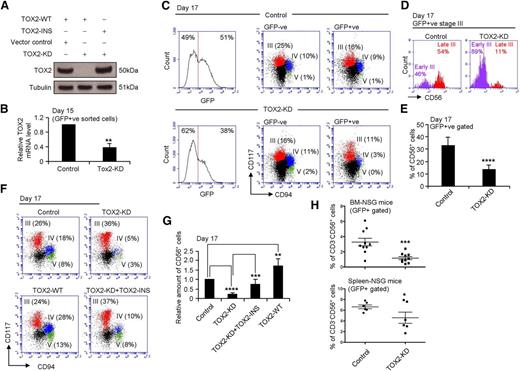
To determine directly whether TOX2 regulates NK cell development, we performed TOX2 knockdown in CD34+ cells. The TOX2 knockdown efficiency of the lentiviral-based TOX2-shRNA construct (TOX2-KD) was assessed by cotransfecting the vector with either the wild-type (TOX2-WT) or the knockdown insensitive mutant construct (TOX2-INS) into 293T cells. TOX2-KD dramatically reduced the protein expression of the WT but not the knockdown insensitive TOX2 mutant (Figure 2A).
TOX2 regulates NK cell maturation. (A) TOX2-WT or TOX2-INS overexpression construct was cotransfected with either control or TOX2-KD construct into 293T cells. TOX2 protein level was analyzed by western blotting. Results are representative of 3 independent experiments. (B) Total RNA was collected from GFP+ sorted developing cells on day 15 for TOX2 RT-qPCR analysis (n = 4). (C) UCB CD34+ cells transduced with control or TOX2-KD constructs were subjected to in vitro NK cell development. Different developmental stages appeared on day 17 and were analyzed by flow cytometry based on CD117 and CD94 staining in both GFP−ve and GFP+ve populations. Results are representative of 3 independent experiments. (D) Stage III (CD117+CD94−) cells from the GFP+ve populations were gated for the analysis of CD56 expression to distinguish early stage III (CD117+CD94−CD56−) and late stage III (CD117+CD94−CD56+) cells. (E) The quantity of CD56+ cells was compared between control and TOX2-KD GFP+ve populations on day 17 by flow cytometry. Bar charts display the mean percentage ± standard deviation (SD) of 8 independent experiments. (F) Different NK developmental stages among control, TOX2-KD–, TOX2-WT–, or TOX2-KD+TOX2-INS–transduced cells (GFP+ve sorted populations) were compared based on the surface expression of CD117 and CD94 by flow cytometry on day 17. FACS plots are representative of 4 independent experiments with (G) the relative production of CD56+ cells shown as the mean ± SD. (H) Control or TOX2-KD CD34+ cells were intravenously injected into irradiated (250 rad) NSG mice (0.5 × 106 cells/mouse; 10 mice per groups). Six weeks after CD34+ cell transplantation, 2.5 μg of RLI by intraperitoneal injection was administered to mice every 5 days for 3 doses. BM and spleen cells were collected 3 days after the last dose of RLI to analyze GFP+CD3−CD56+ NK cell content by flow cytometry. *P < .05, **P < .005, ***P < .001, ****P < .0001.
TOX2 regulates NK cell maturation. (A) TOX2-WT or TOX2-INS overexpression construct was cotransfected with either control or TOX2-KD construct into 293T cells. TOX2 protein level was analyzed by western blotting. Results are representative of 3 independent experiments. (B) Total RNA was collected from GFP+ sorted developing cells on day 15 for TOX2 RT-qPCR analysis (n = 4). (C) UCB CD34+ cells transduced with control or TOX2-KD constructs were subjected to in vitro NK cell development. Different developmental stages appeared on day 17 and were analyzed by flow cytometry based on CD117 and CD94 staining in both GFP−ve and GFP+ve populations. Results are representative of 3 independent experiments. (D) Stage III (CD117+CD94−) cells from the GFP+ve populations were gated for the analysis of CD56 expression to distinguish early stage III (CD117+CD94−CD56−) and late stage III (CD117+CD94−CD56+) cells. (E) The quantity of CD56+ cells was compared between control and TOX2-KD GFP+ve populations on day 17 by flow cytometry. Bar charts display the mean percentage ± standard deviation (SD) of 8 independent experiments. (F) Different NK developmental stages among control, TOX2-KD–, TOX2-WT–, or TOX2-KD+TOX2-INS–transduced cells (GFP+ve sorted populations) were compared based on the surface expression of CD117 and CD94 by flow cytometry on day 17. FACS plots are representative of 4 independent experiments with (G) the relative production of CD56+ cells shown as the mean ± SD. (H) Control or TOX2-KD CD34+ cells were intravenously injected into irradiated (250 rad) NSG mice (0.5 × 106 cells/mouse; 10 mice per groups). Six weeks after CD34+ cell transplantation, 2.5 μg of RLI by intraperitoneal injection was administered to mice every 5 days for 3 doses. BM and spleen cells were collected 3 days after the last dose of RLI to analyze GFP+CD3−CD56+ NK cell content by flow cytometry. *P < .05, **P < .005, ***P < .001, ****P < .0001.
We next transduced UCB CD34+ cells with the GFP-containing TOX2-KD or control construct and then induced the cells to NK cell differentiation in vitro. Persistent TOX2 knockdown was documented on day 15 when we sorted the GFP+ TOX2-KD cell population for RT-qPCR analysis (Figure 2B). GFP-negative and GFP-positive cells were then gated separately for the analysis of different NK cell developmental stages based on the expression of CD117 and CD94 on day 17 of the in vitro cell culture (Figure 2C). Given that TOX2 expression was low or undetectable in early NK development (day 0 to day 7) (supplemental Figure 1B), we hypothesized that TOX2 is not involved in the determination of NK cell commitment. Supporting this hypothesis, TOX2 knockdown did not block the stage III entry of CD34+ cells (Figure 2C). However, the course of subsequent stage transition, notably from early stage III (CD117+CD94−CD56−) to late stage III (CD117+CD94−CD56+), was dramatically impaired in TOX2-deficient cells (Figure 2D). Although there was no difference in the development of stage III (CD117+CD94−), stage IV (CD117+CD94+), and stage V (CD117−CD94+) among the 2 GFP-negative populations (untransduced cells) and the control lentivirus transduced cells, the TOX2-KD population demonstrated a significant reduction in stage IV and stage V populations and also in the number of CD56+ NK cells (Figure 2E). Although there was no difference in cell proliferation rate of GFP+ sorted cells from different developmental stages between control and TOX2-KD samples (supplemental Figure 2A), the negative effect of TOX2-KD on NK cell differentiation was evident at the late time point on day 21 (supplemental Figure 2B-C). These results suggested that TOX2 plays a critical role in stage III to stage IV transition. Although coexpression of TOX2-INS in TOX2-KD CD34+ cells could partially rescue the developmental blockade, overexpression of TOX2-WT in control CD34+ cells significantly enhanced CD56+ NK cell development (Figure 2F-G). These results confirm that TOX2 plays a key role in controlling NK cell maturation.
To confirm this in vitro finding, we tested human NK cell development in vivo by use of a NSG chain immunodeficient mouse model. The mice were transplanted with human CD34+ cells transduced with control or TOX2-KD construct. NK cell differentiation was triggered by injecting the mice with human IL-15 covalently linked to an IL-15R α extended sushi domain (RLI).26,27 Consistent with the in vitro result, NSG mice transplanted with TOX2-KD UCB CD34+ cells developed a significantly lower percentage of CD3−CD56+ NK cells in the BM and spleen (supplemental Figure 3) than those administered control CD34+ cells (Figure 2H).
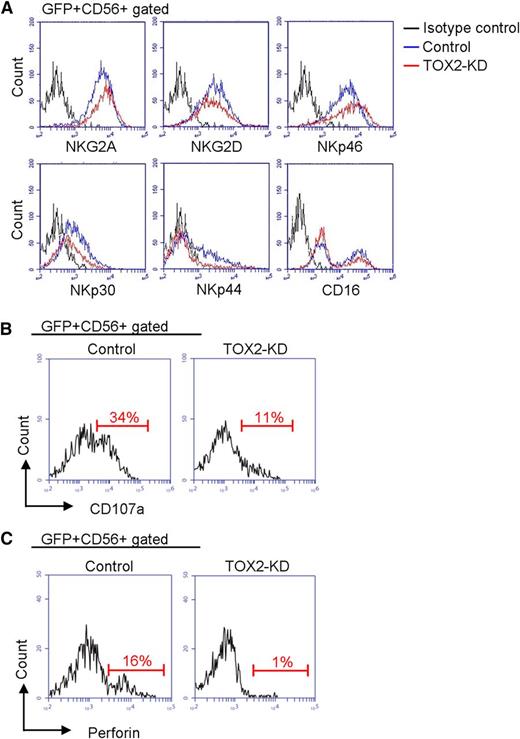
We next compared the GFP+CD56+ cell populations derived from control and TOX2-KD CD34+ cells on day 17 to investigate whether TOX2 deficiency affects NK receptor acquisition during cell maturation. As shown in Figure 3A, TOX2-KD did not appreciably affect the expression of NKG2A, NKG2D, NKp46, or CD16; however, the expression of NKp30 was slightly reduced and that of NKp44 was largely abolished. We also compared the functional response between control and TOX2-KD cells against K562 targets by CD107a mobilization assay. TOX2-KD greatly reduced the degranulation function of NK cells (Figure 3B). We found that the expression of perforin was significantly reduced in TOX2-KD cells. (Figure 3C).
TOX2-KD effects on receptor acquisition, degranulation function, and perforin expression. (A) Control or TOX2-KD–transduced CD34+ cells were subjected to in vitro NK cell differentiation. Flow cytometry was performed on day 17 to analyze the expression of various inhibiting and activating surface receptors (NKG2A, NKG2D, NKp46, NKp30, NKp44, and CD16) on the GFP+CD56+ cell population. Results are representative of 3 independent experiments. (B) CD107a mobilization on K562 stimulation in the GFP+CD56+ cell population was analyzed by flow cytometry. Results are representative of 3 independent experiments. (C) Expression of perforin was compared between control and TOX2-KD GFP+CD56+ cells on day 17 by flow cytometry. Results are representative of 3 independent experiments.
TOX2-KD effects on receptor acquisition, degranulation function, and perforin expression. (A) Control or TOX2-KD–transduced CD34+ cells were subjected to in vitro NK cell differentiation. Flow cytometry was performed on day 17 to analyze the expression of various inhibiting and activating surface receptors (NKG2A, NKG2D, NKp46, NKp30, NKp44, and CD16) on the GFP+CD56+ cell population. Results are representative of 3 independent experiments. (B) CD107a mobilization on K562 stimulation in the GFP+CD56+ cell population was analyzed by flow cytometry. Results are representative of 3 independent experiments. (C) Expression of perforin was compared between control and TOX2-KD GFP+CD56+ cells on day 17 by flow cytometry. Results are representative of 3 independent experiments.
TOX2 directly regulates the expression of T-BET
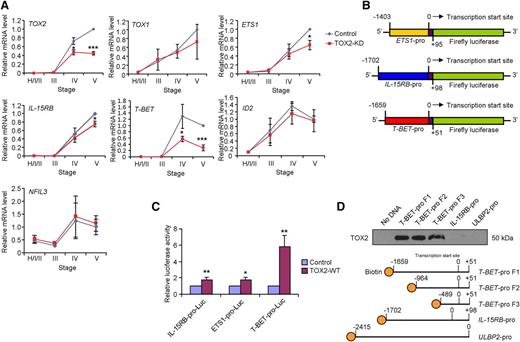
We next investigated if TOX2-KD affected any of the NK cell developmental gene expressions. UCB-derived CD34+ cells that were transduced with control or TOX2-KD lentiviral construct were subjected to in vitro NK cell development. Different developmental stages were sorted for RT-qPCR analysis. A decrease in TOX2 but not TOX1, ID2, and NFIL3 mRNA expression were observed in TOX2-KD cells (Figure 4A). Although there was only a slight decrease of ETS1 and IL-15RB expression in TOX2-KD cells, T-BET expression was substantially reduced in stages IV and V.
TOX2 directly regulates TBX21(T-BET) transcription during NK cell development in vitro. (A) UCB CD34+ cells transduced with control or TOX2-KD constructs were subjected to in vitro NK cell development. Different developmental stages were gated for GFP expression and were sorted as described in Figure 1D. The expression of TOX2 and other NK cell developmental genes was analyzed by RT-qPCR (n = 3). (B) Schematic representation of PGL3-based luciferase reporter constructs containing the putative promoter of ETS1, IL-15RB, or T-BET. (C) IL-15RB, ETS1, or T-BET reporter construct was cotransfected with vector-control or TOX2-WT expression vector into 293T cells. Luciferase activities were determined 3 days after the cotransfection. Data shown are expressed as fold change relative to the luciferase activity observed in vector-control transfected cells ± SD (n = 3). (D) Total cell lysate collected from TOX2-WT–transiently transfected 293T cells was incubated with streptavidin microbeads with or without the biotinylated T-BET-, IL-15RB-, or ULBP2-promoter probe. Protein fractions pulled down by the microbeads were subjected to western blot analysis to detect the presence of TOX2. Data shown are representative of 3 independent experiments. *P < .05, **P < .005, ***P < .0005.
TOX2 directly regulates TBX21(T-BET) transcription during NK cell development in vitro. (A) UCB CD34+ cells transduced with control or TOX2-KD constructs were subjected to in vitro NK cell development. Different developmental stages were gated for GFP expression and were sorted as described in Figure 1D. The expression of TOX2 and other NK cell developmental genes was analyzed by RT-qPCR (n = 3). (B) Schematic representation of PGL3-based luciferase reporter constructs containing the putative promoter of ETS1, IL-15RB, or T-BET. (C) IL-15RB, ETS1, or T-BET reporter construct was cotransfected with vector-control or TOX2-WT expression vector into 293T cells. Luciferase activities were determined 3 days after the cotransfection. Data shown are expressed as fold change relative to the luciferase activity observed in vector-control transfected cells ± SD (n = 3). (D) Total cell lysate collected from TOX2-WT–transiently transfected 293T cells was incubated with streptavidin microbeads with or without the biotinylated T-BET-, IL-15RB-, or ULBP2-promoter probe. Protein fractions pulled down by the microbeads were subjected to western blot analysis to detect the presence of TOX2. Data shown are representative of 3 independent experiments. *P < .05, **P < .005, ***P < .0005.
To validate whether TOX2 directly regulates ETS1, IL-15RB, or T-BET transcription, we selectively cloned 1400 to 1700 bp of the genomic sequence containing the putative promoter region and part of the first exon of ETS1 (−1403 to +95; where 0 was defined as the transcription start site), IL-15RB (−1702 to +98), or T-BET (−1659 to +51) into a luciferase reporter construct (Figure 4B) and then cotransfected with either vector control or TOX2-WT expression construct into 293T cells. As shown in Figure 4C, the expression of TOX2 only slightly modulated the promoter activity of ETS1 and IL-15RB (∼1.7-fold). The coexpression of TOX2, but not the other NK cell development-related transcription factors such as E4BP4, ID2, or VDUP1 (data not shown), significantly increased the T-BET reporter activity (∼sixfold).
We then investigated whether TOX2 directly binds to T-BET promoter region. We designed 3 different 5′-biotinylated PCR primers to amplify different lengths of the T-BET putative promoter and used them in a TOX2 pull-down assay. We also generated a biotinylated-IL15RB promoter and an irrelevant promoter fragment (ULBP2 promoter)28 as a control. As shown in Figure 4D, all of the T-BET promoter fragments but not the IL-15RB and ULBP2 promoter captured TOX2 from the lysates prepared from TOX2-WT-transfected 293T cells, indicating the direct interaction between TOX2 and T-BET promoter region (−489-0).
Overexpression of T-BET but not ETS1 rescues the defect of NK cell development from TOX2-knockdown cells
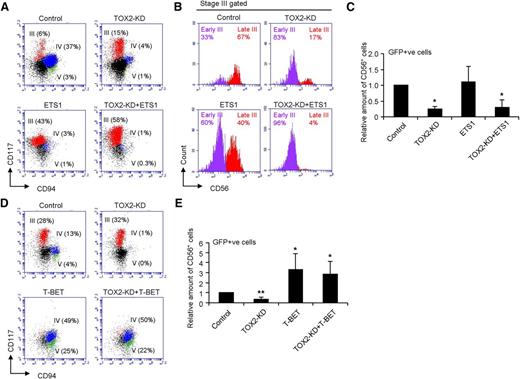
We hypothesized that the regulation of human NK cell maturation by TOX2 is dependent on the expression of T-BET but not the other earlier-stage transcription factors such as ETS1, as we have demonstrated previously that ETS1 is not a target gene of TOX2 because there was only a slight difference in ETS1 mRNA level between control and TOX2-KD during NK cell development (Figure 4A) and that TOX2 resulted in only a moderate effect on ETS1 promoter activity (Figure 4C). Furthermore, ETS1 is known to be a crucial transcription factor dominating the earlier stages of NK cell differentiation.11 We overexpressed ETS1 or T-BET in TOX2-KD-transduced UCB CD34+ cells and then induced the cells to NK cell differentiation (supplemental Figure 4). ETS1 overexpression in control UCB CD34+ cells greatly promoted in vitro NK cell development to late (CD117+CD94−CD56+) stage III cells (Figure 5A-B). However, the increased level of ETS1 could not rescue the blockade of NK cell differentiation from early stage III to late stage III resulted from TOX2 knockdown. NK cell development from TOX2-KD+ETS1 CD34+ cells was similar to that from TOX2-KD cells, which were largely arrested in early stage III (CD117+CD94−CD56−), resulting in the reduced production of CD56+ cells (Figure 5C). T-BET on the other hand, plays important roles in late-stage NK cell maturation.13 Overexpression of T-BET significantly enhanced the production of late developmental stages (stages IV and V) of CD56+ NK cells from both normal and TOX2-KD CD34+ cells (Figure 5D-E). Expression of ID2, another transcription factor that is essential in a later stage of NK cell development,12 in contrast, could not rescue the developmental defect caused by TOX2-KD (data not shown). Collectively, these results confirmed that TOX2 plays a crucial role in stage III transition by directly regulating T-BET expression.
Overexpression of T-BET but not ETS-1 rescues the TOX2 knockdown phenotype in NK cell development. (A) CD34+ cells transduced with control, ETS-1, TOX2-KD, or TOX2-KD+ETS-1 were subjected to in vitro NK cell development. Representative FACS plots of different NK developmental stages based on GFP gating and the surface expression of CD117 and CD94 on day 17 were shown. (B) Stage III (CD117+CD94−) cells were gated for the analysis of CD56 expression to distinguish early stage III (CD117+CD94−CD56−) and late stage III (CD117+CD94−CD56+) cells. (C) The relative amount of GFP+CD56+ cells are calculated: Relative amount of CD56+ cells = (% of GFP+ CD56+ cells of the sample)/(% of GFP+ CD56+ cells of Control) and displayed as the mean ± SD of 5 independent experiments. (D) CD34+ cells transduced with control, T-BET, TOX2-KD, or TOX2-KD+T-BET were subjected to in vitro NK cell development. Representative FACS plots of different NK developmental stages based on GFP gating and the surface expression of CD117 and CD94 on day 17 were shown. (E) The relative amount of GFP+CD56+ cells are displayed as the mean ± SD of 4 independent experiments. *P < .01, **P < .0001.
Overexpression of T-BET but not ETS-1 rescues the TOX2 knockdown phenotype in NK cell development. (A) CD34+ cells transduced with control, ETS-1, TOX2-KD, or TOX2-KD+ETS-1 were subjected to in vitro NK cell development. Representative FACS plots of different NK developmental stages based on GFP gating and the surface expression of CD117 and CD94 on day 17 were shown. (B) Stage III (CD117+CD94−) cells were gated for the analysis of CD56 expression to distinguish early stage III (CD117+CD94−CD56−) and late stage III (CD117+CD94−CD56+) cells. (C) The relative amount of GFP+CD56+ cells are calculated: Relative amount of CD56+ cells = (% of GFP+ CD56+ cells of the sample)/(% of GFP+ CD56+ cells of Control) and displayed as the mean ± SD of 5 independent experiments. (D) CD34+ cells transduced with control, T-BET, TOX2-KD, or TOX2-KD+T-BET were subjected to in vitro NK cell development. Representative FACS plots of different NK developmental stages based on GFP gating and the surface expression of CD117 and CD94 on day 17 were shown. (E) The relative amount of GFP+CD56+ cells are displayed as the mean ± SD of 4 independent experiments. *P < .01, **P < .0001.
Discussion
Previous studies have shown an essential role for TOX1 in murine NK cell development.17 Tox1−/− mice have very few circulating mNK cells with low expression of Id2. Here we demonstrated that knockdown of TOX2 also affected human NK cell differentiation from UCB-derived CD34+ cells in vitro. A similar defect in NK cell development was observed in BM-derived CD34+ cells with TOX2-KD (data not shown). However, the deficiency of TOX2 did not affect the mRNA level and the expression profile of ID2 during NK cell development (Figure 4A). We showed that the expression pattern of TOX2 was fundamentally different from that of TOX1 in which TOX2 appeared in later developmental stages (stages IV and V) whereas a significant amount of TOX1 mRNA was observed by stage III (Figure 1D). Moreover, the expression level of TOX1 remained unchanged in TOX2-KD cells (Figure 4A). Collectively, these results suggest that TOX1 and TOX2 play an independent and sequential role in the regulation of NK cell development.
Our data suggest that TOX2 is not involved in blood NK lineage commitment. RT-qPCR analysis showed that TOX2 mRNA was either low or undetectable from day 0 to day 7 for in vitro NK cell development from UCB-derived CD34+ cells (supplemental Figure 1B). In addition, the silencing of TOX2 gene transcription did not affect the appearance of precursor populations up to early stage III (NKPs) (Figure 2C-D). In line with the finding that TOX2 directly regulates T-BET transcription, T-BET signaling has been reported to be dispensable for the generation of NKPs.13 Studies have shown that T-BET and EOMES play reciprocal roles in maintaining distinct NK cell lineages in the liver and BM in mice.15,16 Although the expression of EOMES is essential to maintain the maturity of NK cells in the BM, NK cell development in the liver requires high levels of T-BET with restricted expression of EOMES. Future investigations in the regulatory role of TOX2 in tissue-specific NK-cell development are warranted.
We found that the expression of perforin was greatly reduced in TOX2-KD cells. Because T-BET has been shown to regulate perforin expression directly,13,16,29 it is likely that the reduced perforin expression was an outcome of decreased T-BET expression in TOX2-KD cells. However, further investigations such as perforin-promoter driven reporter assay are required to rule out whether TOX2 directly controls perforin expression.
In conclusion, we found that TOX2 is highly expressed in stage IV and stage V of mNK but not in the early stages of developing NK cells. In the absence of TOX2, NK cells show substantially reduced T-BET expression, leading to reduced production of mNK cells. This novel knowledge of the molecular events and the mechanism of TOX2 in controlling NK cell development will lead to a better understanding of diseases that are related to NK cell abnormalities.30,31 Our data support future investigation and identification of novel therapeutic agents that upregulate TOX2 expression to promote NK reconstitution after clinical UCB transplantation, as NK cells are important in the control of infections and leukemia relapse.32
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the St. Louis Cord Blood Bank for providing the UCB samples, Dr Geoff Neale for Affymetrix Microarray analysis, and David Galloway for manuscript editing.
This work was supported in part by research grants from the Assisi Foundation of Memphis and Press On Fund.
Authorship
Contribution: Q.P.V. and W.-H.L. designed the study, performed experiments, analyzed data, prepared figures, and wrote the manuscript; J.H. performed the cell-sorting experiments; Y.L. participated in manuscript preparation; B.R. and M.H. participated in flow data analysis; R.A.J.O. provided materials; and W.L. directed the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wing Leung, I3310 MS#1130, 262 Danny Thomas Pl, Memphis, TN 38105; e-mail: wing.leung@stjude.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal