Key Points
Ibrutinib affects collagen and VWF-mediated platelet activation.
The bleeding diathesis correlates with defects in collagen-induced platelet aggregation and firm adhesion on VWF at arterial shear rate.
Abstract
The oral Bruton’s tyrosine kinase inhibitor, ibrutinib, has recently demonstrated high efficiency in patients with relapsed B-cell malignancies. Occurrence of bleeding events has been reported in a subgroup of ibrutinib-treated patients. We demonstrate that ibrutinib selectively inhibits platelet signaling and functions downstream of the collagen receptor glycoprotein VI and strongly affects firm platelet adhesion on von Willebrand factor (VWF) under arterial flow. A longitudinal study of 14 patients indicated a correlation between occurrence of bleeding events and decreased platelet aggregation in response to collagen in platelet-rich plasma and firm adhesion on VWF under arterial flow. The addition of 50% untreated platelets was sufficient to efficiently reverse the effects of ibrutinib, and platelet functions recovered after treatment interruption as physiological platelet renewal occurred. These data have important clinical implications and provide a basis for hemostasis management during ibrutinib treatment.
Introduction
The Bruton tyrosine kinase (Btk) is an essential actor downstream of the B-cell receptor, and its covalent inhibitor, ibrutinib, has recently been approved for therapies of relapsed chronic lymphocytic leukemia (CLL) and mantle cell lymphoma (MCL).1-8 Bleeding has been reported in up to 50% of ibrutinib-treated patients. Most events were grade 1 to 2 (spontaneous bruising or petechiae), but in 5% of patients, they were of grade 3 or higher after trauma.4-6 Platelets are the most important blood cells to prevent bleeding after vascular injury. Two Tec family kinases, Btk and Tec, are involved in platelet activation downstream of the collagen receptor glycoprotein VI (GPVI) via phospholipase Cγ2 (PLCγ2) phosphorylation and activation.9,10 Under arterial shear rate, interaction of platelets with the damaged vessel wall is largely mediated by binding of von Willebrand factor (VWF) to its receptor, the GPIb-IX-V complex. In a mouse model, Btk has been shown to play a role in VWF/GPIb-IX-V-induced platelet activation.11 To further characterize the bleeding events in ibrutinib-treated patients, we investigated the effect of this drug on platelet functions in vitro and ex vivo in 14 patients.
Study design
For in vitro experiments, whole blood, platelet-rich plasma (PRP), or washed platelets from healthy donors free of antiplatelet medication were preincubated with ibrutinib (PCI-32765; Selleckchem) or dimethylsulfoxide for 10 or 30 minutes as indicated. For ex vivo experiments, blood samples were obtained before and 2 to 4 weeks after starting ibrutinib treatment (Imbruvica; Janssen-Cilaq Laboratories) in patients with CLL (420 mg daily) or MCL (560 mg daily)4-6 (French compassionate use program) after informed consent, in accordance with the Declaration of Helsinki. This study was approved by the institutional review board of the Toulouse Hospital. After exclusion of patients with antiplatelet therapy or a platelet count <80 × 109/L, 14 patients were included, the characteristics of whom are reported in supplemental Table 1, available on the Blood Web site. Experiments were performed in PRP prepared from citrated blood (aggregation) or heparinized whole blood (adhesion onto VWF). Most laboratory methods have been described previously12 and are reported in the supplemental Materials. Statistical analysis was performed using the paired or unpaired Student t test (Excel software, *P < .05 and **P < .01).
Results and discussion
Ibrutinib inhibits collagen and collagen-related peptide-induced platelet aggregation and PLCγ2 phosphorylation in vitro
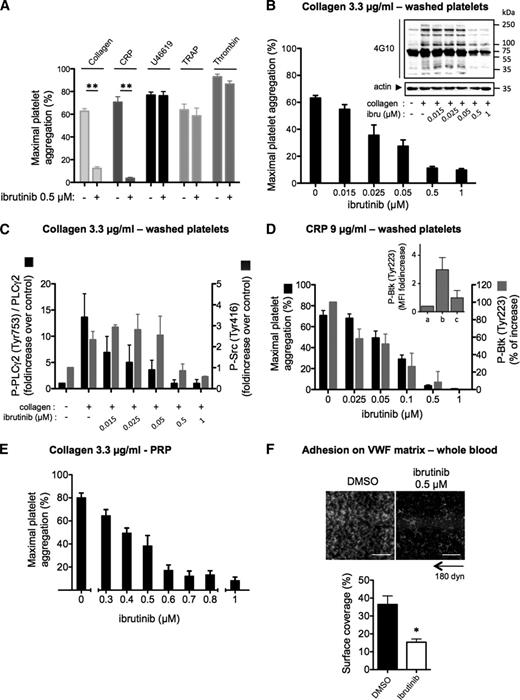
At a clinically achievable dose (0.5 µM),6,13 ibrutinib had no impact on aggregation of washed platelets induced by the thromboxane A2 analog U46619, thrombin-related peptide, or thrombin, but dose-dependently inhibited collagen-induced platelet aggregation (Figure 1A-B; supplemental Figure 1). This effect was accompanied by the inhibition of PLCγ2 phosphorylation on the Btk-dependent phosphorylation site Tyr753.14 Low concentrations of ibrutinib, which strongly reduced PLCγ2 phosphorylation, had no effect on Src family kinase activation (phosphorylation of Tyr416) and on the whole pattern of tyrosine phosphorylation in collagen-stimulated platelets. Higher ibrutinib concentrations (≥0.5 µM) consistently reduced Src family kinase activation and the tyrosine phosphorylation of several proteins (Figure 1B-C). Selectivity tests of ibrutinib against a screening panel of kinases have shown that several Src kinases may indeed be affected at this drug concentration in vitro.3 Ibrutinib dose-dependently inhibited Btk autophosphorylation (Tyr223) and aggregation induced by collagen-related peptide (CRP) in washed platelets (Figure 1D). Ibrutinib also inhibited collagen-induced platelet aggregation in PRP (Figure 1E), with an IC50 value of 0.5 µM, which is nearly the peak dose in plasma of treated patients.6,13 Mixing experiments in PRP showed a correction of the aggregation defect to collagen and Tyr223-Btk autophosphorylation to CRP in direct proportion to untreated platelets (supplemental Figure 3A). This is consistent with the irreversible action of ibrutinib and suggests that platelet transfusion could be an option to correct hemostasis.
Effect of ibrutinib on platelet responses to collagen, CRP, and VWF in vitro. (A) Washed platelets from healthy donors were treated or not with ibrutinib at the indicated concentration for 10 minutes and stimulated with different agonists (collagen, 3.3 µg/mL; CRP, 9 µg/mL; U46619, 5 µM; thrombin receptor-activating peptide, 50 µM; thrombin, 0.5 UI/mL). Platelet aggregation was assessed by turbidimetry and results, expressed as percentage of aggregation, are means ± standard error of the mean (SEM; collagen: n = 12; CRP and other agonists: n = 6). In parallel to aggregation, the effect of ibrutinib on platelet signaling in response to 1 minute stimulation with (B-C) collagen or (D) CRP was assessed by western blotting (whole platelet tyrosine phosphorylation pattern, PLCγ2 phosphorylation on Tyr-753, and Src phosphorylation on Tyr-416) or by flow cytometry for Btk phosphorylation on Tyr-223. (Inset) Fluorescence intensity (MFI) in (a) resting, (b) CRP-stimulated, and (c) CRP-stimulated ibrutinib-treated platelets. Western blots shown are representative of 3 independent experiments. Results of western blot quantification are means ± SEM of 3 to 6 independent experiments. (E) Effect of increasing doses of ibrutinib on collagen-induced platelet aggregation in PRP (n = 4, mean ± SEM). (F) Effect of ibrutinib on platelet adhesion on VWF under arterial flow conditions (4000 s−1 or 180 dyn/cm2) in whole blood. Under these flow conditions, platelet adhesion was dependent on GPIb as verified by its complete inhibition by a monoclonal GPIb antibody (data not shown). Blood from healthy donors was preincubated for 30 minutes with 0.5 µM ibrutinib or dimethylsulfoxide. After 5 minutes of flow, firm platelet adhesion was quantified, after washing with phosphate-buffered saline containing Ca2+/Mg2+ at 4000 s−1 for 1 minute, by measuring the platelet surface coverage values (means ± SEM from 3 independent experiments). *P < .05 and **P < .01. Scale bar: 50 µm.
Effect of ibrutinib on platelet responses to collagen, CRP, and VWF in vitro. (A) Washed platelets from healthy donors were treated or not with ibrutinib at the indicated concentration for 10 minutes and stimulated with different agonists (collagen, 3.3 µg/mL; CRP, 9 µg/mL; U46619, 5 µM; thrombin receptor-activating peptide, 50 µM; thrombin, 0.5 UI/mL). Platelet aggregation was assessed by turbidimetry and results, expressed as percentage of aggregation, are means ± standard error of the mean (SEM; collagen: n = 12; CRP and other agonists: n = 6). In parallel to aggregation, the effect of ibrutinib on platelet signaling in response to 1 minute stimulation with (B-C) collagen or (D) CRP was assessed by western blotting (whole platelet tyrosine phosphorylation pattern, PLCγ2 phosphorylation on Tyr-753, and Src phosphorylation on Tyr-416) or by flow cytometry for Btk phosphorylation on Tyr-223. (Inset) Fluorescence intensity (MFI) in (a) resting, (b) CRP-stimulated, and (c) CRP-stimulated ibrutinib-treated platelets. Western blots shown are representative of 3 independent experiments. Results of western blot quantification are means ± SEM of 3 to 6 independent experiments. (E) Effect of increasing doses of ibrutinib on collagen-induced platelet aggregation in PRP (n = 4, mean ± SEM). (F) Effect of ibrutinib on platelet adhesion on VWF under arterial flow conditions (4000 s−1 or 180 dyn/cm2) in whole blood. Under these flow conditions, platelet adhesion was dependent on GPIb as verified by its complete inhibition by a monoclonal GPIb antibody (data not shown). Blood from healthy donors was preincubated for 30 minutes with 0.5 µM ibrutinib or dimethylsulfoxide. After 5 minutes of flow, firm platelet adhesion was quantified, after washing with phosphate-buffered saline containing Ca2+/Mg2+ at 4000 s−1 for 1 minute, by measuring the platelet surface coverage values (means ± SEM from 3 independent experiments). *P < .05 and **P < .01. Scale bar: 50 µm.
Ibrutinib inhibits platelet adhesion onto VWF under high shear rate in vitro
Binding of VWF to GPIb-V-IX is required for tethering platelets to the injured vessel wall and for integrin-mediated platelet arrest under blood flow. VWF-GPIb interaction stimulates cytoskeletal reorganization in rolling platelets via a shear-sensitive signaling pathway linked to PLC and intracellular calcium mobilization.15 Preincubation of blood from healthy donors with ibrutinib decreased the firm platelet adhesion onto VWF under high shear rate (Figure 1F) while sparing platelet rolling (supplemental Video 1) and expression of GPIb (supplemental Figure 2A).
Hemostasis-related adverse effects of ibrutinib in CLL and MCL patients correlate with platelet dysfunction
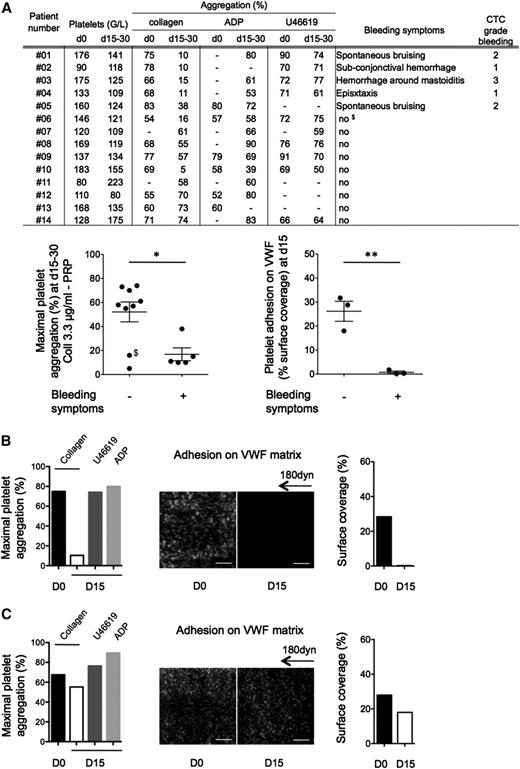
From 14 included patients, 5 displayed treatment-related hemostasis defects including spontaneous bruising, superficial bleedings, or epistaxis (Figure 2A), whereas all had a clinical response to the drug (supplemental Table 1). Standard light transmission aggregometry in PRP indicated that ibrutinib treatment had no or a very minor effect on adenosine 5′-diphosphate- or U46619-induced platelet aggregation in all patients tested. Seven patients presented a significant defect in platelet aggregation to collagen. All patients with bleeding diathesis presented a strong inhibition of collagen-induced aggregation in PRP. The firm platelet adhesion onto VWF was analyzed in 6 patients. Platelets from patients with bleeding hardly adhered onto VWF under flow compared with patients with no bleeding symptoms (Figure 2A-C).
Platelet functions of CLL and MCL patients treated with Imbruvica (ibrutinib). (A) Clinical and biological data were collected before initiation of the treatment (day 0) and after 2 to 4 weeks of treatment (days 15-30). Platelet aggregation induced by collagen (3.3 µg/mL), adenosine 5′-diphosphate (5 µM), or U46619 (5 µM) on PRP was assessed as in Figure 1 at day 0 and days 15 to 30 and expressed as means ± SEM. It is noteworthy that platelet aggregation induced by collagen at day 0 was slightly reduced in the patients group compared with healthy donors (88 ± 6% for healthy donors and 69 ± 7% for patients, P < .01, n = 19 healthy donors). Of note, in the nonbleeding group at days 15 to 30, patients 06 and 10 had a strong reduction of aggregation. Importantly, patient 06 ($) experienced a severe metrorrhagia at day 50. Platelet adhesion on VWF matrix was assessed for 6 patients (01, 02, 03, 07, 08, and 14) as in Figure 1 and expressed as the surface coverage. Results are presented as means ± SEM. (B) Example of a patient (01) showing platelet dysfunction and bleeding symptoms on ibrutinib treatment. (C) Example of a patient (08) with normal platelet responses and no hemostasis-related adverse effect on ibrutinib treatment. Hyperleukocytosis was not correlated to ex vivo platelet dysfunction. *P < .05 and **P < .01. D0, day 0; D15, day 15.
Platelet functions of CLL and MCL patients treated with Imbruvica (ibrutinib). (A) Clinical and biological data were collected before initiation of the treatment (day 0) and after 2 to 4 weeks of treatment (days 15-30). Platelet aggregation induced by collagen (3.3 µg/mL), adenosine 5′-diphosphate (5 µM), or U46619 (5 µM) on PRP was assessed as in Figure 1 at day 0 and days 15 to 30 and expressed as means ± SEM. It is noteworthy that platelet aggregation induced by collagen at day 0 was slightly reduced in the patients group compared with healthy donors (88 ± 6% for healthy donors and 69 ± 7% for patients, P < .01, n = 19 healthy donors). Of note, in the nonbleeding group at days 15 to 30, patients 06 and 10 had a strong reduction of aggregation. Importantly, patient 06 ($) experienced a severe metrorrhagia at day 50. Platelet adhesion on VWF matrix was assessed for 6 patients (01, 02, 03, 07, 08, and 14) as in Figure 1 and expressed as the surface coverage. Results are presented as means ± SEM. (B) Example of a patient (01) showing platelet dysfunction and bleeding symptoms on ibrutinib treatment. (C) Example of a patient (08) with normal platelet responses and no hemostasis-related adverse effect on ibrutinib treatment. Hyperleukocytosis was not correlated to ex vivo platelet dysfunction. *P < .05 and **P < .01. D0, day 0; D15, day 15.
This effect appeared reversible as shown in 1 patient (03) who interrupted ibrutinib therapy due to an infection complication. The recovery of collagen-induced platelet aggregation paralleled the expected physiological platelet renewal rate with nearly half-maximal and complete aggregation restored 60 and 168 hours after treatment interruption, respectively (supplemental Figure 3B).
Clinical implication
Our results complement 2 previous short notes16,17 and show that, in vitro, ibrutinib specifically affects GPVI- and GPIb-mediated platelet functions. Besides Btk, ibrutinib can also inhibit Tec (in vitro IC50 = 78 nM),3 suggesting that inhibition of these 2 important kinases downstream of these platelet receptors9-11 may be responsible for the observed effect. X-linked agammaglobulinemia patients, deficient in Btk, exhibit only a weak platelet aggregation defect in response to low doses of collagen and no bleeding phenotype,9,18 probably because Tec compensates the lack of functional Btk.10 The concentration of ibrutinib required to inhibit 50% of collagen-induced-platelet aggregation in PRP in our study approximates the peak concentration of the drug in humans.6,13 Polymorphisms or drug interactions on ibrutinib metabolism leading to pharmacokinetics or pharmacodynamic variations as well as redundant platelet signaling pathways and variations in GPVI and GPIb expression in patients19 may contribute to explain that only a subset of treated patients display spontaneous bleeding. The combined action of ibrutinib on GPVI and GPIb pathways likely explains the defect in primary hemostasis, particularly the bleeding in the microvasculature where the shear rate is elevated. Consistent with this, a recent study confirms the occurrence of mild bleeding episodes in 44% of ibrutinib-treated CLL patients.20 Our study also suggests that platelet transfusion at a dose sufficient to get 50% of fresh platelets may correct hemostasis in emergency, provided it is given after elimination of ibrutinib from blood, which takes several hours after last intake.6 By analogy, treatment interruption for 5 days may be sufficient before an invasive procedure at high bleeding risk.
Although 1 patient with reduced aggregation to collagen had no bleeding manifestation, this test seems to be indicative of an increased bleeding risk in patients under ibrutinib treatment as also suggested by a very recent clinical study.21 Further prospective studies will determine whether it is useful for guiding the therapeutic strategy, especially for patients under antiplatelet therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank A. Zakaroff and C. Pecher (I2MC) for their help in flow cytometry analysis, the imaging facility of I2MC, and M. P. Gratacap and S. Severin for critical reading of the manuscript.
This work was supported by grants from INSERM, Fondation Pour la Recherche Médicale, and the Cancer Pharmacology of Toulouse Oncopole & Region (CAPTOR) project (Investissements d’Avenir ANR11-PHUC001).
Authorship
Contribution: M.L., E.D., C.G., and P.-A.L. designed and performed most experiments and analyzed data; S.C. performed flow cytometric analysis; J.-C.B. contributed to platelet aggregation tests; A.-S.M. and L.Y. selected patients; and M.L., C.T., P.S., L.Y. and B.P. designed research, supervised the work, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bernard Payrastre, INSERM U1048, I2MC, 1 Avenue Jean Poulhés, BP 84225, 31432 Toulouse Cedex 04, France; e-mail: bernard.payrastre@inserm.fr; or Loic Ysebaert, Service d’Hématologie IUCT-Oncopôle; e-mail: ysebaert.loic@iuct-oncopole.fr.
References
Author notes
E.D., C.G., and P.-A.L. contributed equally to this work.
L.Y. and B.P. contributed equally to this work.