Abstract
The last 6 decades have seen major advances in the understanding of immunologic diseases, driven by preclinical animal models. Indeed, bone marrow transplantation (BMT) has its genesis in rodent models dating back to the 1950s. Allogeneic BMT and its major complication, graft-versus-host disease (GVHD), represent a paradigm for the translation of preclinical concepts into clinical practice. The appreciation that GVHD can be thought of as a stepwise escalation in immune activation characterized by eventual massive target tissue apoptosis has allowed the design of rational approaches to better manage patients. Here, we describe the pathophysiology of GVHD as defined in preclinical models, focusing on the successes and failures of this research to instruct and translate clinical practice. We also provide a commentary on the limitations of these models so that they may be better appreciated and addressed in future studies. Notable preclinical successes include the definition of modern immune suppression, reductions in conditioning intensity, posttransplant cyclophosphamide, and the promotion of regulatory T-cell reconstitution. New strategies including naïve T-cell depletion, focused cytokine and chemokine inhibition, and the blockade of costimulation now also appear highly promising and very likely to translate into patients in the near future.
Introduction
Allogeneic peripheral blood stem cell or bone marrow transplantation (hereafter referred to as BMT) continues to expand in use as curative therapy for hematologic malignancies, predominantly as a vehicle for generating potent immunologic graft-versus-leukemia (GVL) effects. Modern BMT has its genesis in the early 1950s from the original rodent studies demonstrating the ability of bone marrow to prevent radiation lethality.1,2 As early as 1956, a GVL effect was mooted,3 but it was not until 1968 that the first successful allogeneic BMT was performed.4 Improvements in donor and recipient selection, conditioning regimens, graft-versus-host disease (GVHD) prophylaxis and treatment, and attention to infectious complications mean that, in 2014, the majority of transplant-related mortality is due to relapse of primary malignancy. However, GVHD remains a major cause of treatment failure, resulting in mortality in 20% of recipients.5 A recent study reviewed transplant outcome between 1993 and 1997, and compared these to more recent outcomes in the period 2003 through 2007.6 Considering all indications for transplant, 70% of patients developed grade II-IV acute GVHD (aGVHD) in the latter cohort, a slight decrease compared with 77% in the earlier group. This small improvement reflects the reduction in incidence of severe (grade III/IV) GVHD which decreased from 30% to 14% in the 2003 to 2007 cohort. Data pertaining to chronic GVHD are more elusive, but it is estimated that 60% to 80% of long-term survivors of BMT experience some degree of chronic GVHD.7 More recent studies reintroducing T-cell–depleting antibodies in the peritransplant period have demonstrated further reductions in the incidence of significant GVHD; however, these reductions have not been associated with improvements in survival.8 Thus, at a time of intense activity in experimental modeling of GVHD and GVL, only modest improvements in clinical outcome are being realized.
So what insights have been gained from these preclinical studies? Is it too early yet to see the effects of this work translated, are there scientific limitations of these studies to date that limit their clinical relevance, or is the translation to a relatively small discipline too difficult? Here, we propose that, although all of the above apply to some extent, experimental studies have made major contributions to clinical practice over the last 3 decades that are not broadly appreciated.
This review will focus on the pathophysiology of GVHD and, in particular, the contributions made by experimental models to the understanding of GVHD and the clinical practice of transplantation. In addition, we will highlight the limitations of experimental systems in the hope that some of these issues can be better appreciated and addressed scientifically as the field moves forward. Finally, we will outline recently defined pathways of GVHD that hold particular promise for clinical translation.
Animal models of GVHD: in a nutshell
The majority of animal models currently used to study GVHD use inbred mice. The advantages of murine systems include the ability to: (1) control environmental conditions (including microbiota and conditioning regimens), (2) control major histocompatibility complex (MHC) and minor histocompatibility antigen disparities, (3) transplant large numbers of recipients simultaneously, (4) genetically mutate key molecules in donor and/or recipients and within specific cell populations, (5) image and define immune reactions in real time and at multiple time points, (6) test lead therapeutics in a relevant disease model, and (7) the utilization of a relatively cost-effective model. The GVHD that develops in these systems is in response to alloantigen, as in patients, and on the whole faithfully mirrors the GVHD seen in the clinic. Furthermore, the ability to strictly control multiple variables enhances scientific rigor and facilitates the dissection of mechanisms of disease. However, humans are not inbred; they come to transplant as genetically diverse individuals, at varying ages, with varying diseases in varying states of remission, and with varying exposure to many, often toxic, previous therapies. Although it is easy to blame these differences for the failure of many preclinical mouse concepts to be emulated in patients, appreciating the potential limitations of the initial preclinical and/or the subsequent clinical study is more often informative. In particular, it should be noted that most murine models of GVHD (regardless of whether they are MHC matched or not) induce early CD4-dependent GVHD that is characterized by inflammatory cytokine dysregulation and T helper 1 (Th1) differentiation.9 This degree of CD4 dependence is unlikely to be as dominant in patients receiving HLA-matched grafts and immune suppression with calcineurin inhibitors. Indeed, the absence of immune suppression in murine models has the capacity to identify pathways of GVHD in mice that may well not even be operative clinically and this is a fundamental limitation of these systems. An additional weakness is the almost exclusive use of total body irradiation (TBI) in animal systems, which is increasingly disparate to clinical practice. Preclinical studies should thus ideally analyze both CD4- and CD8-dependent GVHD, and this rather than the use of MHC disparate or “clinically relevant” MHC matched models is paramount. It is no surprise then that seminal studies leading to the generation of our current immune suppression regimens come from studies in canines in the 1980s.10 These canine studies continue to this day and provide a robust preclinical model to analyze the therapeutic efficacy of therapeutics, allowing their prioritization for subsequent clinical trials. In particular, canine models overcome the issue of inbred backgrounds and better permit the analysis of therapies concurrent with clinically relevant immune suppression, antibiotics, etc. Their weakness is their cost and lack of genetic manipulation, meaning that murine and canine systems are likely to continue to perform complementary functions in the modeling of GVHD.
Animal models of GVL: many limitations
The ultimate goal of allogeneic BMT is to separate GVHD and GVL, which may ultimately require genetic manipulation of the T-cell receptor (TCR) to ensure specificity for recipient leukemia or at least hematopoiesis, an approach now feasible.11 Nevertheless, even small shifts in favor of GVL are advantageous, as demonstrated by improved outcome in studies using peripheral blood stem cell grafts as opposed to bone marrow.12 This may be possible by targeting potential inflammatory molecules that mediate GVHD13 but are dispensable for the cognate interaction and cytotoxicity between cytotoxic T lymphocyte and leukemia that appears to be a prerequisite for GVL responses.14 The experimental GVHD literature demonstrates numerous therapeutic maneuvers that appear to achieve this aim. However, there remain a number of caveats to these studies that relate to limitations in the experimental GVHD systems and leukemia targets used. As noted, many of the therapeutic interventions published have demonstrated efficacy in CD4-dependent GVHD models (inflammatory cytokine inhibition as an example) but the GVL systems used experimentally are largely a measure of GVL that is CD8-dependent.15,16 Thus, the separation of the 2 processes may be overestimated due to the differential pathways used by GVHD and GVL in these systems. This is, of course, only an issue if the same does not hold true clinically. Although we do not yet have a complete understanding of the relative importance of class I– vs class II–dependent GVHD in the clinic when both pathways are operative, it appears that experimental models overestimate CD4-dependent pathways relative to those in the clinic.9
The second issue relates to the leukemia targets used in experimental studies. The vast majority of animal systems have used cell lines on the recipient strain background to study GVL. Some of these cell lines (particularly B-cell lymphoma lines) grow variably in vivo and are exquisitely sensitive to a GVL effect such that they rarely survive in the presence of any alloreactive T-cell response. Thus, many of these studies to date demonstrate that a GVL effect remains following a given therapeutic intervention, but it is not possible to ascertain the quality of that response. Second, it is clear that different cell lines are sensitive to different killing pathways17 (likely the case in human leukemia also), meaning that multiple targets sensitive to killing by the pathway in question should ideally be tested in vivo. Third, it is becoming clear that primary leukemia (generated by retroviral insertion of appropriate oncogenes) may model GVL in a more realistic and clinically relevant fashion than cell lines.14,18 Strikingly different results in experimental studies of regulatory T cells (Tregs) are a case in point.19,20 For all these reasons, it seems clear that the use of cell lines in mouse models of GVHD to date has generated data that probably underestimate the effect of therapeutic interventions on the quality of GVL responses and this is likely an inherent weakness in murine systems. However, studying GVHD and GVL that require similar CD4- or CD8-dependent pathways while using primary leukemia rather than cell lines will help to generate data that faithfully model clinical GVL.
aGVHD: breaking it down into 3 phases
The characteristic pathologic feature of aGVHD is target tissue (skin, liver, gastrointestinal [GI] tract) apoptosis. Although idiopathic pneumonia syndrome is increasingly viewed as a manifestation of aGVHD in the lung, apoptosis is generally not a feature. aGVHD was initially described as a “cytokine storm” involving a three-step disease process by Ferrara and Deeg.21 These steps involve: (1) transplant conditioning and associated inflammation, (2) donor T-cell priming and differentiation, and (3) an effector phase of tissue apoptosis mediated by inflammatory cytokines and cellular (T and NK cell) effectors. It still remains useful to break GVHD down into these phases, particularly when considering where advances in the field of clinical transplantation originate, and where the limitations in this paradigm arise.
(1) Transplant conditioning and inflammation
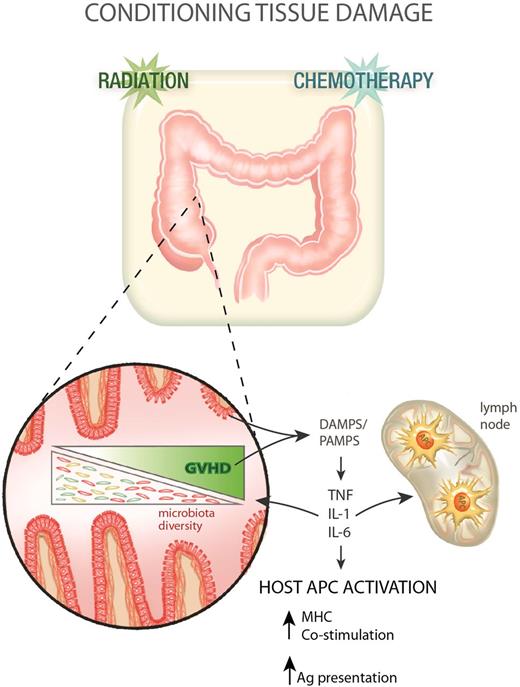
Clinical allogeneic stem cell transplantation has traditionally involved the use of myeloablative chemotherapy, with or without TBI as a cytoreductive and immunosuppressive preparative regimen. For technical reasons largely relating to access and reproducibility, almost all preclinical animal studies use TBI alone as conditioning. In the mid 1990s, it became clear that the intensity of conditioning was an important factor in determining the tempo and nature of subsequent GVHD. Mouse models were instrumental in demonstrating the mechanism by which pretransplant conditioning contributes to GVHD pathophysiology (Figure 1).22-24 Many groups demonstrated that intensity of TBI and chemotherapy used during conditioning dictated the integrity of the GI mucosa and the subsequent transfer of bacterial lipopolysaccharide22-25 and other “danger/pathogen-associated molecular patterns” (DAMPS/PAMPS) into the systemic circulation. Indeed, the role of the microbiota in promoting GVHD has been a central theme in the last 4 decades of BMT research and more recent data has demonstrated profound effects of GVHD on the modification of microbiota diversity that promotes the GVHD response.26,27 It is thus now clear that lipopolysaccharide is but one of many microbiome-derived products that drive GVHD.26,28 The characteristic proinflammatory cytokines released by recipient cells at this early stage of GVHD pathogenesis are tumor necrosis factor (TNF) and interleukins 1 and 6 (IL-1 and IL-6).22,23,29,30 This inflammation has 2 potential effects. First, it activates recipient antigen-presenting cells (APCs), enhancing the ability of professional APCs to prime T cells and also likely promoting antigen presentation by nonprofessional APCs within tissue.31 Second, these cytokines may provide complimentary costimulatory signals to donor T cells (eg, TNF, IL-6).13,32 Finally, they generate tissue inflammation, allowing the access of T cells to GVHD target tissue.33
GVHD pathophysiology phase 1: transplant conditioning and inflammation. Following conditioning (radiation and/or chemotherapy), the integrity of the GI mucosa becomes compromised allowing the release of DAMPS and PAMPS, which in turn promote the production of proinflammatory cytokines from recipient cells. These cytokines contribute to host APC (hematopoietic and nonhematopoietic) activation in the gut and lymphoid tissue. GVHD impacts on the gut microbiota, reducing its diversity with a loss of enteric commensal organisms and an outgrowth of pathogenic microbes that further exacerbates the pathological DAMP/PAMP cascade.
GVHD pathophysiology phase 1: transplant conditioning and inflammation. Following conditioning (radiation and/or chemotherapy), the integrity of the GI mucosa becomes compromised allowing the release of DAMPS and PAMPS, which in turn promote the production of proinflammatory cytokines from recipient cells. These cytokines contribute to host APC (hematopoietic and nonhematopoietic) activation in the gut and lymphoid tissue. GVHD impacts on the gut microbiota, reducing its diversity with a loss of enteric commensal organisms and an outgrowth of pathogenic microbes that further exacerbates the pathological DAMP/PAMP cascade.
Thus, strategies to reduce GVHD at the conditioning phase have focused on a: (1) reduced-intensity conditioning (RIC), (2) manipulation of the microbiota, and (3) the blockade of inflammatory cytokines that promote the downstream priming T cells and their entry to tissue (discussed in section 3).
Canine models of allogeneic BMT were first used to address concerns that decreasing the intensity of the conditioning regimen would result in graft rejection (enhanced host-versus-graft effects) and loss of GVL effects. In the 1990s, these models confirmed that GVHD was reduced with so-called RIC protocols,34 and that poor engraftment could be overcome by the addition of peritransplant immune suppression, defining the combination of cyclosporine and mycophenolate that is used today.34-36 Murine data at this time confirmed the ability and mechanism by which conditioning intensity dictated the severity of GVHD.23 Because the toxicity of myeloablative regimens generally prevented elderly patients from receiving transplants, the clinical introduction of RIC was rapid and the first reports were published in 1997 and 1998.37,38 Although numerous conditioning regimens are now in clinical practice, the use of total lymphoid irradiation and anti-thymocyte globulin deserves special mention. This protocol was pioneered in animal models and shown to prevent GVHD but not GVL by enrichment of an IL-4–secreting recipient natural killer (NK) T-cell subset.39 This protocol has been successfully translated to the clinic with outcomes as predicted by animal models.40 Today, approximately one-third of patients receive transplants after RIC, a procedure that had its genesis in animal models. Attempts to improve GI tract integrity after myeloablative conditioning with cytokines such as IL-11 and KGF were promising approaches based on animal models24 but found limited therapeutic success due to either toxicity or incomplete efficacy in humans.41,42
The relationship between the GI tract microbiota and GVHD was first reported in 197428 and the clinical importance and translation has been exemplified by the ability of gut decontamination to attenuate GVHD clinically.43 The current era of bacterial multidrug resistance severely limits this approach but it is likely that manipulation of the microbiota to a more tolerogeneic phenotype will be possible in the near future, as demonstrated in recent animal models.44 Clearly, the increased understanding of the influence of conditioning on GVHD gleaned from mouse models has had an enormous influence on clinical BMT in the modern era.
(2) Donor T-cell priming and differentiation
Priming of donor T cells
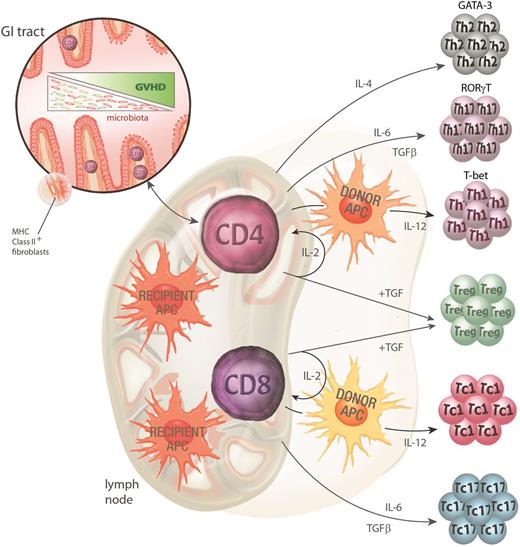
The priming and activation of donor T cells is the hallmark of the second phase of GVHD pathogenesis. The process requires the interaction of the donor T cell with an APC-presenting alloantigen (usually a minor histocompatibility antigen) which drives T-cell activation and differentiation (Figure 2). The site and type of APC involved in the initiation of GVHD has been the focus of a great deal of investigation in murine systems over the last 15 years. Current data support the notion that donor CD8 T cells are predominantly activated by recipient hematopoietic APCs whereas donor CD4 T cells can also be activated by recipient nonhematopoietic APC within the GI tract.31,45,46 Donor APCs also contribute to GVHD, once donor T cells are primed by recipient APCs.47 Studies using stringent conditional ablation of individual APC subsets have demonstrated that no single-recipient APC subset is mandatory in this process. In particular, despite the long-held and frequently quoted paradigm, recipient dendritic cells (DCs) are not required to initiate GVHD and may even regulate subsequent donor T-cell expansion.31,48 Although recipient DCs appear important in the generation of Treg responses,49 we have demonstrated that DCs can attenuate GVHD induced by alloantigen-specific TCR-transgenic effector T cells via a process of deletion.31 In this scenario, Tregs do not expand and so the process can also occur in the absence of effects on Tregs. In addition, both recipient B cells50 and macrophages51,52 appear capable of regulating T-cell expansion and aGVHD. It should, however, be noted that donor DCs would appear the critical donor APCs in regard to alloantigen presentation and the maintenance of GVHD.53-55 It is now clear that in the presence of GVHD, donor DCs have an impaired ability to present exogenous antigen, particularly via MHC class II, and that this contributes to poor immune function in patients with GVHD.56 The level of immune incompetence during GVHD had been historically attributed to defects within the T-cell compartment alone, and it will now be important to consider both the donor DC and T cell in immune-restorative therapy during GVHD.56
GVHD pathophysiology phase 2: donor T-cell priming and differentiation. Donor CD4 T cells contained within the graft are activated by the inflammatory milieu early after conditioning, facilitating their rapid access to the gut and lymphoid tissue. Once in the gut, MHC class II–expressing recipient nonhematopoietic APCs can initiate priming to host antigens while recipient hematopoietic APC initiate priming in lymphoid tissue. Recipient hematopoietic APCs appear to be the dominant APCs for CD8 T-cell priming. Donor APCs can further contribute to this priming process. Activation in the presence of various cytokines (particularly IL-4, IL-6, and IL-12) instructs T-cell differentiation along specific lineage pathways (type 2, type 17, and type 1, respectively). The transcription factors GATA-3, RORγt, and T-bet are critical for these Th2, Th17, and Th1 differentiation pathways. Tregs are differentiated in the presence of IL-2 and TGFβ (in the absence of IL-6) and abrogate the differentiation of effector T cells via effects on DCs and effector T cells themselves.
GVHD pathophysiology phase 2: donor T-cell priming and differentiation. Donor CD4 T cells contained within the graft are activated by the inflammatory milieu early after conditioning, facilitating their rapid access to the gut and lymphoid tissue. Once in the gut, MHC class II–expressing recipient nonhematopoietic APCs can initiate priming to host antigens while recipient hematopoietic APC initiate priming in lymphoid tissue. Recipient hematopoietic APCs appear to be the dominant APCs for CD8 T-cell priming. Donor APCs can further contribute to this priming process. Activation in the presence of various cytokines (particularly IL-4, IL-6, and IL-12) instructs T-cell differentiation along specific lineage pathways (type 2, type 17, and type 1, respectively). The transcription factors GATA-3, RORγt, and T-bet are critical for these Th2, Th17, and Th1 differentiation pathways. Tregs are differentiated in the presence of IL-2 and TGFβ (in the absence of IL-6) and abrogate the differentiation of effector T cells via effects on DCs and effector T cells themselves.
The costimulatory signals between the APC and T cell have been the subject of intense preclinical study over the last 25 years. In particular, blockade of the CD40-CD154 and CD80/CD86-CD28/CTLA4 axes have been explored in an attempt to induce alloantigen-specific T-cell anergy and tolerance (reviewed in Kinnear et al57 ). Clinical reagents that inhibit the CD80/CD86-CD28/CTLA4 axis are now available (belatacept, abatacept) and have proven to be effective, nontoxic alternatives to calcineurin inhibitors in renal transplantation, although in humans these agents do not appear to induce the tolerance seen in rodents.57 These agents are just beginning to be explored in clinical BMT and may be of particular value in patients intolerant of calcineurin inhibitors.
Donor T-cell expansion and differentiation
Once primed by alloantigen, donor T cells undergo proliferation and differentiation such that they secrete a predictable suite of cytokines and/or gain cytolytic function. This process of generating terminally differentiated effector T cells is the principal determinant of GVHD. Mouse studies have clearly demonstrated that it is the priming and differentiation of naïve (rather than memory) T cells that results in GVHD58,59 and early human data support this paradigm.60,61 This raises the possibility that depletion of naïve T cells may prevent GVHD while allowing the transfer of donor memory T cells that may provide pathogen-specific immunity. This concept is currently being tested clinically in the United States. The proliferation and expansion of donor T cells requires IL-2 and its autocrine binding via the IL-2 receptor complex (CD25 and CD122). The mainstay of both GVHD prophylaxis and treatment is calcineurin inhibitors and steroids, respectively, both of which decrease the IL-262 available to drive effector T-cell proliferation and survival. aGVHD, particularly that targeting the GI tract, has been defined as a Th1-mediated disease and the classical Th1-derived cytokines (eg, interferon γ [IFNγ] and TNF) are systemically elevated and clearly mediate target organ pathology in preclinical models.23,24,63,64 Donor Th1 differentiation, characterized by expression of the transcription factor t-bet, is driven in these systems by IL-12 secretion from APCs and this cytokine (together with IL-23) is now a target for neutralization in clinical BMT given the availability of clinical-grade inhibitors. It is becoming clear that GVHD within individual organs may be generated by different T-cell differentiation programs. In particular, it has been noted that Th17 differentiation tends to result in GVHD within the skin and lung.65-67 Th17 differentiation, characterized by expression of the transcription factor RORγt, is induced by IL-6 (in the presence of transforming growth factor β [TGFβ], IL-21, and IL-23) which is secreted by numerous cell types including APCs. IL-6 inhibition is now being tested in phase 2-3 trials in clinical BMT and efficacy of this approach should be available shortly. Donor Th17 cells secrete IL-17A, IL-17F, IL-21, and IL-22, most of which have been shown to be pathogenic in preclinical systems.66,68-70 Th2 differentiation, characterized by secretion of IL-4 and expression of the transcription factor GATA3, may also be involved in GVHD, preferentially within liver and skin.67,71 In general, CD8 T cells can also differentiate along similar pathways although to date, research has focused only on T cytotoxic (Tc)-1 and Tc2 differentiation (although there is growing interest in the Tc17 lineage) and these cells appear to have differential ability to induce aGVHD and GVL.66,72 In particular, Tc2 cells appear to offer a favorable profile with regard to their ability to mediate GVL rather than GVHD.72 A more recently described follicular helper T-cell (TFH) subset is characterized by expression of the transcription factor bcl6 and secretion of IL-21. TFH cells appear to be important in generating germinal center (B-cell) reactions and alloantibody that is involved in the manifestations of chronic GVHD, particularly within the lung.73 IL-21 derived from TFH and Th17 cells thus appears an attractive target to prevent both acute and chronic GVHD.66,70
The identification of Tregs, characterized by expression of the transcription factor FoxP3 and induced by IL-2 and TGFβ, has led to an explosion in interest in this cell subset for the induction of tolerance. It is clear from preclinical and more recent clinical studies that both acute and particularly chronic forms of GVHD are characterized by major quantitative and qualitative defects in Tregs.74,75 Therapeutic approaches to harness the regulatory potential of these cells have revolved around strategies to enhance their expansion in vivo, or to select large numbers (with or without in vitro expansion), for adoptive transfer. It became clear very early that clinical calcineurin-based immune suppression prevented Treg differentiation and expansion, a phenomenon not seen with the mammalian target of rapamycin inhibitor, rapamycin.76 This has led to trials of rapamycin both in clinical GVHD prophylaxis and treatment protocols and for the expansion of Tregs in vitro.77 Approaches to expand Tregs in vivo have focused on their preferential requirement for IL-2 and indeed exogenous low-dose IL-2 results in a marked expansion of Tregs and improvement in chronic GVHD in a proportion of patients.78 In addition, it is now possible to purify small numbers of Tregs based on CD25 selection that are nevertheless sufficient to allow profound modulation of effector T cells when transferred after haplomismatched BMT,79 an approach translated from the preclinical systems pioneered by the Negrin laboratory.76 Alternatively, large numbers of Treg can be expanded in vitro based on culture systems using IL-2 and TGF (± rapamycin)80 and the ability of these cells to modulate GVHD is the subject of intense clinical investigation. Additional novel approaches to expand Tregs using histone deacetylase (HDAC) inhibitors have also recently translated from animal models into clinical practice.81 The administration of demethylating agents after experimental BMT also has promising effects on the induction of Tregs.82 It is worth noting that IL-10 and TGFβ are major immunoregulatory cytokines produced by Treg and T-regulatory (Tr)-1 (FoxP3neg IL-10 producing) regulatory cells and IL-10 polymorphisms were among the first genetic polymorphisms to be correlated with disease outcome in GVHD.83 Approaches to enhance Treg reconstitution after BMT now represent the most promising strategy to prevent and treat GVHD and demonstrate the ability of preclinical research to guide clinical therapeutic intervention.
Cytokine administration to enhance thymopoiesis and improve immune reconstitution after T-cell–depleted transplantation is an attractive proposition supported by numerous preclinical studies (reviewed in Dudakov and van den Brink84 ). The cytokines KGF, IL-7, and IL-22 in particular appear promising and some have entered clinical testing.84,85 The use of these agents in T-cell–replete transplantation, however, remains more problematic because IL-7 and IL-22 at least may also promote GVHD.68,69
Although the use of standard immune suppression potently inhibits donor T-cell expansion and differentiation, the fact that at least half of patients still develop GVHD demonstrates that standard therapy is less than perfect. Perhaps one of the most interesting therapeutic approaches to prevent alloreactive donor T-cell expansion is the administration of high doses of cyclophosphamide early after haplomismatched BMT to recipients otherwise receiving no immune suppression. This approach has been developed in animal models for over 4 decades (reviewed in Luznik et al86 ) and results in efficient deletion of alloreactive effector T cells while sparing Tregs.87 The translation of this approach from mice to patients has been strikingly successful88 and has permitted increased BMT activity over the last 5 years in patient groups previously untreatable. The administration of bortezomib in the peritransplant period also promotes alloreactive T-cell depletion in vivo and has also generated promising results in phase 2 studies89 that have been translated from earlier animal studies.
(3) The effector phase
aGVHD
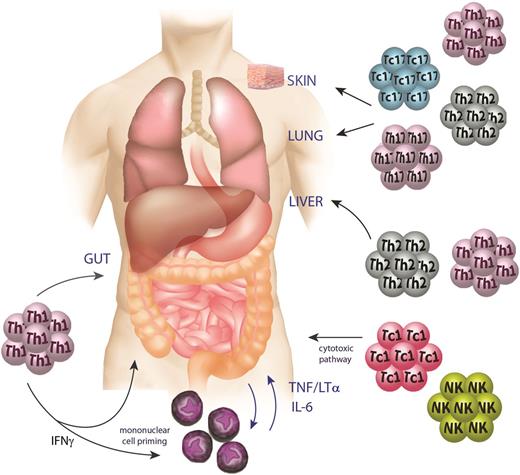
The cumulative effect of transplant conditioning and inflammation, the subsequent promotion of alloantigen presentation, and T-cell priming and differentiation leads to an alarming immune response in which inflammatory cytokines and cytolytic T and NK cells mediate antigen-independent and -dependent killing of target tissues (Figure 3). The movement of T cells into target organs is mediated by chemokines and the recent clinical studies blocking CCR5 have demonstrated promising results that are consistent with the earlier animal studies.90 It has become clear over the last decade that inflammatory cytokines (particularly TNF and IL-1), largely the result of CD4-dependent GVHD, can induce target tissue apoptosis independent of any cognate (ie, TCR-MHC II) interaction.30 The inflammatory cytokines so characteristic of aGVHD are derived from both monocytes/macrophages and T cells themselves and cytokines from the latter clearly amplify cytokine dysregulation in the former.24 In contrast, target tissue injury in CD8-dependent GVHD does appear to primarily require cognate interaction between T cells and the target tissue (ie, TCR-MHC II).14 In this way, the cytolytic machinery within the T cell may invoke apoptosis in adjacent cells via perforin and granzyme molecules,91 TNF, and the Fas/FasL signaling pathway.92-94 Importantly, these same T cells use perforin,95 TNF-related apoptosis-inducing ligand,96 and TNF16 mediated cytolytic pathways for their GVL effects (where both CD4 and CD8 T cells require cognate TCR-MHC interactions with target leukemia)14,97 and so targeting these pathways has generally been deleterious on GVL effects. Nevertheless, these pathways may be viable targets for the treatment of severe GVHD, where GVL effects may have already effectively eradicated residual leukemia.
GVHD pathophysiology phase 3: the effector phase. During the effector phase of GVHD, inflammatory cytokines (IFNγ, TNF, lymphotoxin, and IL-6) derived from macrophages and T cells mediate apoptosis in target tissues, particularly within the gut. Donor Th1/Tc1, Th2/Tc2, and Th17/Tc17 cells elicit GVHD with relatively tissue-specific patterns mediated in part by their respective chemokine profiles and the relative sensitivity of the target to effector cytokines generated by each lineage. Cytolytic T and NK cells mediate antigen-dependent killing of target tissues via the perforin/granzyme and TNF member pathways.
GVHD pathophysiology phase 3: the effector phase. During the effector phase of GVHD, inflammatory cytokines (IFNγ, TNF, lymphotoxin, and IL-6) derived from macrophages and T cells mediate apoptosis in target tissues, particularly within the gut. Donor Th1/Tc1, Th2/Tc2, and Th17/Tc17 cells elicit GVHD with relatively tissue-specific patterns mediated in part by their respective chemokine profiles and the relative sensitivity of the target to effector cytokines generated by each lineage. Cytolytic T and NK cells mediate antigen-dependent killing of target tissues via the perforin/granzyme and TNF member pathways.
Perhaps the dominant effector of GVHD identified in preclinical models is that of the TNF family (eg, TNF and lymphotoxin), especially in GVHD of the GI tract.16,17,32,98,99 Clinical evidence for the importance of TNF in GVHD was initially obtained in studies of donor and recipient genetic polymorphisms, which established that high donor TNF production and increased recipient responses to TNF via receptor signaling contributed to GVHD risk.100 The importance of the TNF signaling pathway in clinical BMT has been suggested by the apparent efficacy of TNF blocking agents in the prevention and treatment of GVHD.101-104 However, TNF is not present in serum of patients receiving immune suppression, in contrast to mice that do not, leading groups to use the soluble TNF receptor-1 as a surrogate.105 Furthermore, a recent study by the Blood and Marrow Transplant Clinical Trials Network suggested that TNF inhibition had limited efficacy as an adjunct to steroids (and calcineurin inhibition) in the treatment of aGVHD.106 This again highlights the fact that although significant efficacy may be seen following single-agent therapeutic intervention in animal models, concurrent standard clinical therapies may have already modified this pathway.
IL-1 is a potent proinflammatory cytokine that is important in animal models of GVHD and a large number of other acute and chronic inflammatory diseases.107,108 Early studies in the mouse demonstrated that IL-1 is an important mediator of GVHD and preclinical studies suggested that this molecule would be a target for clinical therapy.107,109 However, a phase 3 trial involving a large number of patients indicated that IL-1 blockade was insufficient to prevent GVHD, nor could it impact toxicity or overall survival.110 Given this data and the paradoxical effects of IL-18 in GVHD,111 although the inflammasome may be involved in GVHD in animal models,112 it would appear an unattractive target in clinical BMT. Likewise, it is clear that IFNγ plays complex, organ-specific, pathogenic and protective effects in GVHD63 and is inhibited by calcineurin inhibition113 such that it appears a complex therapeutic target in isolation.
Animal models clearly demonstrate IL-6 as a major mediator of GVHD with both direct effects on target tissue and important effects on Th17 differentiation.13,114 Importantly, clinical data does suggest that IL-6 is dysregulated in BMT patients,115 making it a more attractive target, currently under investigation in clinical trials.
Chronic GVHD
Our understanding of the mechanisms of chronic GVHD is very limited. Because chronic GVHD is characterized by end-organ fibrosis, it is of no surprise that the effector pathways differ from those mediating aGVHD at both a cellular and molecular level. However, like aGVHD, it also appears that differing pathways of disease operate within individual target organs. Certainly fibrosis in the skin appears to be an IL-17–dependent process66 whereas aberrant B-cell function and alloantibody deposition appear important in lung and liver.73 Whether the latter process also involves Th17 and/or TFH differentiation remains to be determined. Certainly preclinical studies have shown TGFβ, derived from tissue macrophages, as a major mediator of chronic GVHD.116 However, the critical role for TGFβ in regulatory responses make therapeutic inhibition a double-edged sword. Strategies for the prevention of chronic GVHD will thus likely need to focus on an aberrant pathway of T-cell differentiation or the final common initiating cell, putatively macrophages and/or B and plasma cells.
Conclusions
Over the last 60 years, animal models have played a critical role in shaping our understanding of GVHD and GVL responses and this is perhaps their greatest contribution to the field. Nonetheless, they have also generated major new paradigms that have instructed our clinical practice and examples of these contributions are shown in Table 1. As we move forward, it is useful to consider where the next advances will come from and how we should assess the potential of a therapeutic intervention defined in a preclinical study to translate into clinical practice. There are some obvious criteria and these are shown in Table 2. Based on these criteria, we suggest that therapeutics aimed at Treg reconstitution, alloreactive and naive T-cell depletion, and IL-6, IL-12p40, and IL-21 inhibition are the most immediately promising candidates and should be a high priority for clinical trials.
Examples of the concepts generated in animal models
| Concepts generated . |
|---|
| Established clinical procedures |
| BMT |
| GVL |
| T-cell depletion |
| Peritransplant immune suppression |
| Donor lymphocyte infusions |
| RIC |
| TLI-based conditioning |
| Posttransplant cyclophosphamide |
| Promising concepts under investigation |
| Treg expansion (eg, adoptive transfer, IL-2, HDAC inhibition, demethylating agents) |
| Naïve T-cell depletion |
| Alternative alloreactive T-cell depletion strategies (eg, bortezomib) |
| Cytokine inhibition (eg, IL-6, IL-12, IL-21) |
| Chemokine blockade (CCR5) |
| CAR-expressing donor lymphocyte infusions |
| Costimulation blockade |
| Manipulation of microbiota |
| Concepts generated . |
|---|
| Established clinical procedures |
| BMT |
| GVL |
| T-cell depletion |
| Peritransplant immune suppression |
| Donor lymphocyte infusions |
| RIC |
| TLI-based conditioning |
| Posttransplant cyclophosphamide |
| Promising concepts under investigation |
| Treg expansion (eg, adoptive transfer, IL-2, HDAC inhibition, demethylating agents) |
| Naïve T-cell depletion |
| Alternative alloreactive T-cell depletion strategies (eg, bortezomib) |
| Cytokine inhibition (eg, IL-6, IL-12, IL-21) |
| Chemokine blockade (CCR5) |
| CAR-expressing donor lymphocyte infusions |
| Costimulation blockade |
| Manipulation of microbiota |
CAR, chimeric antigen receptor; TLI, total lymphoid irradiation.
Predicting the translation of preclinical therapeutic strategies
| Will a new therapeutic strategy translate into the clinic? . |
|---|
| 1. Is the pathway in question still active in patients (or animals) receiving immune suppression? If not, does preclinical evidence suggest the inhibition of the pathway in isolation will be feasible and more efficacious than current immune suppression? |
| 2. Is the effect large (complete) rather than incremental (partial)? |
| 3. Does the intervention inhibit GVHD at multiple stages of its pathophysiology, and preferably early? |
| 4. Is the pathway active in both class I– and class II–dependent GVHD? If not, it should at least not have opposing effects in CD8 and CD4 T-cell–dependent GVHD. |
| 5. Are there potential toxicities in humans that might reasonably not be evident in the animal model? |
| 6. Are there detrimental effects of the intervention on GVL and pathogenic-specific immunity and if so is this manageable and outweighed by the potential benefits in treating GVHD? |
| 7. Bearing in mind that pharmaceutical companies have minimal interest in BMT as a sole market, is the pathway operative in other disease settings such that the generation and development of reagents to the clinic is likely to be commercially viable? |
| Will a new therapeutic strategy translate into the clinic? . |
|---|
| 1. Is the pathway in question still active in patients (or animals) receiving immune suppression? If not, does preclinical evidence suggest the inhibition of the pathway in isolation will be feasible and more efficacious than current immune suppression? |
| 2. Is the effect large (complete) rather than incremental (partial)? |
| 3. Does the intervention inhibit GVHD at multiple stages of its pathophysiology, and preferably early? |
| 4. Is the pathway active in both class I– and class II–dependent GVHD? If not, it should at least not have opposing effects in CD8 and CD4 T-cell–dependent GVHD. |
| 5. Are there potential toxicities in humans that might reasonably not be evident in the animal model? |
| 6. Are there detrimental effects of the intervention on GVL and pathogenic-specific immunity and if so is this manageable and outweighed by the potential benefits in treating GVHD? |
| 7. Bearing in mind that pharmaceutical companies have minimal interest in BMT as a sole market, is the pathway operative in other disease settings such that the generation and development of reagents to the clinic is likely to be commercially viable? |
Acknowledgments
The authors acknowledge Madeleine Flynn, Queensland Institute of Medical Research Berghofer Medical Research Institute, for assistance with the illustrations. K.A.M. is a National Health and Medical Research Council Clinical Training Fellow, K.P.A.M. is a Cancer Council Queensland Senior Research Fellow, and G.R.H. is a National Health and Medical Research Council Australia Fellow and a Queensland Health Senior Clinical Research Fellow.
Authorship
Contribution: K.A.M., K.P.A.M., and G.R.H. all contributed to the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Geoffrey R. Hill, QIMR Berghofer Medical Research Institute, 300 Herston Rd, Herston, QLD, Australia, 4006; e-mail: geoff.hill@qimrberghofer.edu.au.