Innate lymphoid cells (ILCs) are lymphoid cells that do not express rearranged receptors and have important effector and regulatory functions in innate immunity and tissue remodeling. ILCs are categorized into 3 groups based on their distinct patterns of cytokine production and the requirement of particular transcription factors for their development and function. Group 1 ILCs (ILC1s) produce interferon γ and depend on Tbet, group 2 ILCs (ILC2s) produce type 2 cytokines like interleukin-5 (IL-5) and IL-13 and require GATA3, and group 3 ILCs (ILC3s) include lymphoid tissue inducer cells, produce IL-17 and/or IL-22, and are dependent on RORγt. Whereas ILCs play essential roles in the innate immune system, uncontrolled activation and proliferation of ILCs can contribute to inflammatory autoimmune diseases. In this review, we provide an overview of the characteristics of ILCs in the context of health and disease. We will focus on human ILCs but refer to mouse studies if needed to clarify aspects of ILC biology.
Introduction
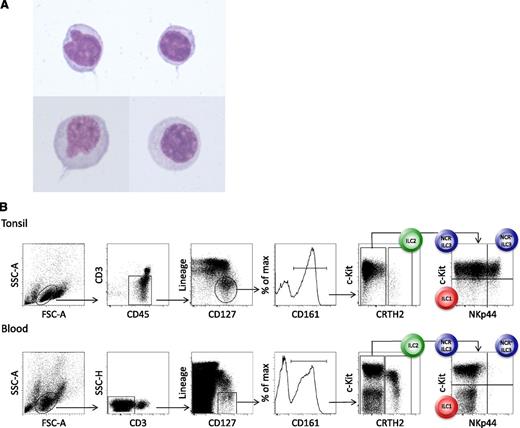
Innate lymphoid cells (ILCs) constitute a recently identified family of mononuclear hematopoietic cells with key functions in the preservation of epithelial integrity and tissue immunity throughout the body. They are defined by their lymphoid morphology (Figure 1A) and the absence of rearranged antigen-specific receptors.1
Morphology and phenotype of peripheral blood ILCs. (A) May-Grünwald-Giemsa staining (original magnification ×100), after cytospin, of human lineage− CD127+ CRTH2+ ILC2s that were sort-purified from the peripheral blood. (B) Phenotype and gating strategy for ILC1s, ILC2s, and NCR− and NCR+ ILC3s derived from tonsil (upper panels) and peripheral blood (lower panels) of healthy humans. The lineage cocktail contains markers for T cells (TCRαβ and TCRγδ), B cells (CD19), NK cells (CD94), myeloid and plasmacytoid dendritic cells (CD1a, CD11c, CD123, and BDCA2), monocytes and macrophages (CD14), mast cells (FcεR1), and stem cells (CD34). In the peripheral blood, NCR+ ILC3s are virtually absent in healthy individuals.
Morphology and phenotype of peripheral blood ILCs. (A) May-Grünwald-Giemsa staining (original magnification ×100), after cytospin, of human lineage− CD127+ CRTH2+ ILC2s that were sort-purified from the peripheral blood. (B) Phenotype and gating strategy for ILC1s, ILC2s, and NCR− and NCR+ ILC3s derived from tonsil (upper panels) and peripheral blood (lower panels) of healthy humans. The lineage cocktail contains markers for T cells (TCRαβ and TCRγδ), B cells (CD19), NK cells (CD94), myeloid and plasmacytoid dendritic cells (CD1a, CD11c, CD123, and BDCA2), monocytes and macrophages (CD14), mast cells (FcεR1), and stem cells (CD34). In the peripheral blood, NCR+ ILC3s are virtually absent in healthy individuals.
Two prototypic members of the ILC family are natural killer (NK) cells and lymphoid tissue inducer (LTi) cells. NK cells were discovered in the mouse in 19752 and are operationally defined by the capability to kill certain target cells in the absence of antigen-specific priming. LTi cells, identified in 1997,3 are essential for the formation of lymph nodes during embryogenesis and are also present in the postnatal gut, where they are important for the formation of cryptopatches and intestinal lymphoid structures, also called isolated lymphoid follicles. NK cells and LTi cells are developmentally related, as both cell types require the common γ (γc) chain of the interleukin-2 (IL-2) receptor and the transcriptional repressor Id2 for their development.4
Recently, several distinct ILC populations have been identified that also depend on the γc chain5,-7 and Id2 for their development.6,8 Each of these ILC populations show distinct patterns of cytokine production that mirror the cytokine-secreting profiles of helper T-cell subsets.9 Recent studies demonstrated that ILC populations have important effector functions during the early stages of the immune response against microbes,5,6 in tissue repair,10,11 in the anatomical containment of commensals,12 and in maintaining epithelial integrity at barrier surfaces.13 ILC function needs to be tightly regulated, as uncontrolled activation and proliferation can contribute to severe inflammation and damage in gut,14 lung,15,16 skin,17,18 and liver.19
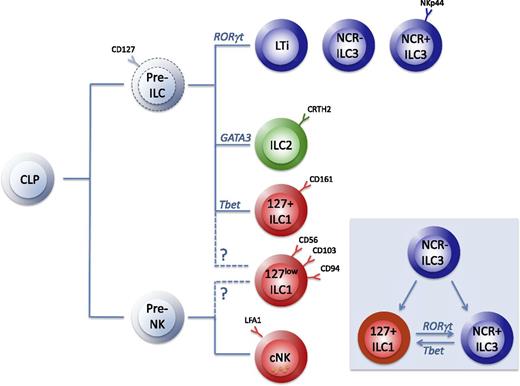
A group of researchers has proposed a classification of these ILC populations on the basis of their phenotypical (Figure 1B) and functional characteristics.20 The nomenclature is based on the helper T-cell nomenclature and categorizes the ILC subsets into 3 groups (Figure 2): group 1 ILCs (ILC1s) comprise ILCs that produce interferon γ (IFN-γ); group 2 ILCs (ILC2s) produce type 2 cytokines, in particular IL-5 and IL-13; and group 3 ILCs (ILC3s) produce Il-17 and/or IL-22. In this model, NK cells were classified in group 1 ILCs because of their capacity to produce IFN-γ.20 Recent information on the developmental pathways of mouse ILCs, however, suggests that NK cells and CD127+ ILCs should be considered as the innate forms of CD8+ and CD4+ T cells, respectively.21 Development of NK cells and CD127+ ILCs may be driven by the transcription factors Nfil322 and GATA3,23,24 respectively (Figure 2). In this review, we provide an overview of the characteristics of distinct ILC subsets, with emphasis on human ILCs. Because of space constraints, we will not extensively review human LTi cells but refer to reviews that are published elsewhere.1,25 In addition, some excellent recent reviews provide more detailed information on mouse ILC biology.26,27
The developmental relationship of ILCs. The NK cell progenitor (pre-NK) and the ILC progenitor (pre-ILC) evolve from the common lymphoid progenitor (CLP), but the phenotype and developmental requirements of the pre-ILCs have not been defined in humans (dotted lines). ILC3s and ILC2s develop from pre-ILCs under the influence of the transcription factors RORγt and GATA3, respectively. CD127+ ILC1s may derive from pre-ILCs or may be developmentally separated as part of the NK branch together with conventional NK cells (cNK) and CD127low ILC1s. Inset: ILCs have plasticity, as RORγt+ NCR− ILC3 can differentiate in vitro into ILC1s and into NCR+ ILC3s; the latter, in turn, can be induced into a NKp44− cKit− CRTH2− ILC1s, and vice versa, depending on specific activation signals. Whether these ILC1s are similar to the NKp44− cKit− CRTH2− ILC1s that can be found in human tissues and blood remains to be determined. During these processes, these cells downregulate RORγt and upregulate Tbet.
The developmental relationship of ILCs. The NK cell progenitor (pre-NK) and the ILC progenitor (pre-ILC) evolve from the common lymphoid progenitor (CLP), but the phenotype and developmental requirements of the pre-ILCs have not been defined in humans (dotted lines). ILC3s and ILC2s develop from pre-ILCs under the influence of the transcription factors RORγt and GATA3, respectively. CD127+ ILC1s may derive from pre-ILCs or may be developmentally separated as part of the NK branch together with conventional NK cells (cNK) and CD127low ILC1s. Inset: ILCs have plasticity, as RORγt+ NCR− ILC3 can differentiate in vitro into ILC1s and into NCR+ ILC3s; the latter, in turn, can be induced into a NKp44− cKit− CRTH2− ILC1s, and vice versa, depending on specific activation signals. Whether these ILC1s are similar to the NKp44− cKit− CRTH2− ILC1s that can be found in human tissues and blood remains to be determined. During these processes, these cells downregulate RORγt and upregulate Tbet.
Key features of group 1, 2, and 3 ILCs
ILC1s are not yet well defined. Both in humans and mice, several populations have been described that produce IFN-γ but not other signature cytokines such as IL-17, IL-22, or IL-5. The developmental relationship of these ILC1s is not yet clarified, and the mouse equivalents of currently identified human ILC1s and vice versa are not precisely known. Human group 1 ILCs include 2 ILC1 subsets that can be distinguished from NK cells and each other based on their intestinal anatomical location and the expression of certain surface markers. Intraepithelial CD127low ILC1s express CD103, CD56, CD94, and NKp44 and are responsive to IL-12 and IL-15.28 The intraepithelial CD127low ILC1s bear resemblance to NK cells, as these cells are CD56+ and also express perforin, which is essential for cytotoxicity.28 The equivalent of CD127low ILC1s in the mouse are claimed to be intraepithelial CD160+ ILCs (CD160 is also expressed in human CD127low ILC1s), which, like NK cells, are dependent on the transcription factor Nfil3 but, in contrast to NK cells, are independent of IL-15 for their development.28 It has been argued that the CD127low ILC1s are the equivalents of intraepithelial CD8+ T cells,28 which would fit with the emerging concept that Nfil3-dependent ILCs are innate equivalents of CD8+ T cells.21 Human CD127high ILC1s are predominantly located in the lamina propria; lack CD56, CD94, and NKp44 expression; and respond to IL-12 and IL-18 by producing IFN-γ.29 It is likely that human CD127high ILC1s depend on IL7 for their development, but this has yet to be confirmed.
Both human CD127low and CD127high ILC1 populations highly express Tbet, and it is likely that Tbet is important for development. Recently, a Tbet-dependent ILC1 population was identified in the mouse liver that displays some cytotoxic activity30 but differs from NK cells by lacking expression of the transcription factor Eomes and develops from a precursor that is unable to differentiate into NK cells.21,30,31 The human equivalent of these cells has yet to be identified.
ILC2s are responsive to IL-25, IL-33,6,32,-34 and thymic stromal lymphopoietin (TSLP)15,35 and produce type 2 cytokines, predominantly IL-5 and IL-13, but also amphiregulin, which is important for tissue repair,11 and IL-9 and IL-4. GATA3 is essential for development and function of human ILC2, whereas Notch signaling stimulates development of human ILC2s.36 Likewise, mouse ILC2s are dependent on GATA335,37 and Notch in addition to TCF-1 and RORα38,39 for their development and function.
ILC3s respond to IL-235,7,40,-42 and IL-1β43,44 by secreting IL-22. ILC3s can be divided into natural cytotoxicity receptor (NCR)+ (NKp46 in mice and NKp44 and NKp46 in humans) and NCR− ILC3s. In the mouse, ILC3s depend on the transcription factors Notch, TCF-1, and RORγt for their development and function, but the requirement of these factors for human ILC3 development has yet to be confirmed. Mouse ILC3s that express the NCR NKp46 also require Tbet for their development,45,-47 but it is unknown whether this is also the case for human NKp44+ ILC3s. It has been observed that upon stimulation with IL-1β and IL-23, human RORγt+ NKp44− ILC3s can differentiate in vitro into NCR+ ILC3s and under the influence of IL-12 into CD127+ ILC1s.29 NCR+ ILC3s can also differentiate into ILC1s upon stimulation with IL-12. During this process, these cells downregulate RORγt and upregulate Tbet.29 A similar transition of NCR+ ILC3s into INF-γ–producing Tbethigh RORγtlow ILCs has been observed in vivo in a mouse model.45 These data indicate that ILC3s are plastic cells that can adopt an ILC1 fate depending on environmental cues. An overview of the ILC subsets, their phenotype, the signature cytokines they produce, their critical transcription factors, and their developmental relationships is provided in Tables 1 and 2 and Figure 2.
Phenotype of human ILCs
| . | NK cells . | ILC1 . | ILC2 . | ILC3 . | |||
|---|---|---|---|---|---|---|---|
| CD127− . | CD127− . | CD127+ . | LTi . | NKp44− . | NKp44+ . | ||
| Lin | − | − | − | − | − | − | − |
| CD7 | + | + | + | + | + | + | |
| CD16 | +/− | − | − | − | − | − | − |
| CD25 | +/− | − | + | + | − | ||
| CD56 | + | + | − | − | − | − | +/− |
| CD94 | + | − | − | − | − | − | − |
| CD103 | + | − | |||||
| CD117 (cKit) | − | − | +/− | low | + | + | |
| CD127 | − | − | + | + | + | + | + |
| CD161 | +/− | + | + | +/− | + | + | |
| CCR6 | − | + | + | + | + | + | |
| CRTH2 | − | − | + | − | − | − | |
| ICOS | + | ||||||
| IL1bR | − | − | + | + | + | + | + |
| IL12RB | + | + | − | − | − | +/− | |
| IL15RA | + | + | − | ||||
| IL17RB | − | − | + | − | − | ||
| IL23R | − | +/− | − | + | + | + | |
| MHC-II | +/− | +/− | +/− | ||||
| NKp44 | +/− | + | − | − | − | − | + |
| NKp46 | + | + | − | − | low | low | |
| ST2 | +/− | − | + | − | − | ||
| . | NK cells . | ILC1 . | ILC2 . | ILC3 . | |||
|---|---|---|---|---|---|---|---|
| CD127− . | CD127− . | CD127+ . | LTi . | NKp44− . | NKp44+ . | ||
| Lin | − | − | − | − | − | − | − |
| CD7 | + | + | + | + | + | + | |
| CD16 | +/− | − | − | − | − | − | − |
| CD25 | +/− | − | + | + | − | ||
| CD56 | + | + | − | − | − | − | +/− |
| CD94 | + | − | − | − | − | − | − |
| CD103 | + | − | |||||
| CD117 (cKit) | − | − | +/− | low | + | + | |
| CD127 | − | − | + | + | + | + | + |
| CD161 | +/− | + | + | +/− | + | + | |
| CCR6 | − | + | + | + | + | + | |
| CRTH2 | − | − | + | − | − | − | |
| ICOS | + | ||||||
| IL1bR | − | − | + | + | + | + | + |
| IL12RB | + | + | − | − | − | +/− | |
| IL15RA | + | + | − | ||||
| IL17RB | − | − | + | − | − | ||
| IL23R | − | +/− | − | + | + | + | |
| MHC-II | +/− | +/− | +/− | ||||
| NKp44 | +/− | + | − | − | − | − | + |
| NKp46 | + | + | − | − | low | low | |
| ST2 | +/− | − | + | − | − | ||
ILCs are categorized into CD127− NK cells, CD127− ILC1s and CD127+ ILC1s, ILC2s, and ILC3s, the latter including LTi, NCR− ILCs, and NCR+ ILC3s. +/− denotes expression that is upregulated by ILCs after activation or by a nonactivated subset of ILCs.
Signature cytokines of human ILCs
| . | NK cells . | ILC1 . | ILC2 . | ILC3 . | |||
|---|---|---|---|---|---|---|---|
| CD127− . | CD127− . | CD127+ . | LTi . | NKp44− . | NKp44+ . | ||
| Amphiregulin | − | − | − | + | − | − | − |
| BAFF | + | ||||||
| CSF1 | + | ||||||
| GM-CSF | +/− | + | + | ||||
| Granzyme | + | + | − | − | − | − | − |
| IFN-γ | + | + | + | − | − | − | − |
| IL-2 | + | ||||||
| IL-3 | + | ||||||
| IL-4 | − | − | + | − | − | − | |
| IL-5 | − | − | + | − | − | − | |
| IL-8 | + | ||||||
| IL-9 | − | − | + | − | − | − | |
| IL-13 | − | − | + | − | − | +/− | |
| IL-17 | − | − | − | + | + | − | |
| IL-21 | + | ||||||
| IL-22 | − | − | +/− | − | + | + | |
| LTα | − | + | |||||
| LTβ | − | + | |||||
| Perforin | + | + | − | − | − | − | − |
| TNFα | + | ||||||
| . | NK cells . | ILC1 . | ILC2 . | ILC3 . | |||
|---|---|---|---|---|---|---|---|
| CD127− . | CD127− . | CD127+ . | LTi . | NKp44− . | NKp44+ . | ||
| Amphiregulin | − | − | − | + | − | − | − |
| BAFF | + | ||||||
| CSF1 | + | ||||||
| GM-CSF | +/− | + | + | ||||
| Granzyme | + | + | − | − | − | − | − |
| IFN-γ | + | + | + | − | − | − | − |
| IL-2 | + | ||||||
| IL-3 | + | ||||||
| IL-4 | − | − | + | − | − | − | |
| IL-5 | − | − | + | − | − | − | |
| IL-8 | + | ||||||
| IL-9 | − | − | + | − | − | − | |
| IL-13 | − | − | + | − | − | +/− | |
| IL-17 | − | − | − | + | + | − | |
| IL-21 | + | ||||||
| IL-22 | − | − | +/− | − | + | + | |
| LTα | − | + | |||||
| LTβ | − | + | |||||
| Perforin | + | + | − | − | − | − | − |
| TNFα | + | ||||||
ILCs are categorized into CD127− NK cells, CD127− ILC1s and CD127+ ILC1s, ILC2s, and ILC3s, the latter including LTi, NCR− ILCs, and NCR+ ILC3s. +/− denotes cytokine expression by a subset of ILCs after activation for example with phorbol 12-myristate 13-acetate (PMA)/ionomycin.
GM-CSF, granulocyte-macrophage colony-stimulating factor.
Developmental relationship of ILCs and NK cells
Because human CD127+ ILCs express many NK cell markers including CD56, NKp44, and CD161,5,40 questions about the relationship between NK cells and CD127+ ILCs have been raised. The developmental pathway of NK cells in the mouse has been studied in detail and has been reviewed extensively recently,48 but the developmental pathways of human NK cell development and the transcription factors that control this process are less well defined.49,50 Earlier studies have led to a model in which 4 stages of human NK cell development can be distinguished on the basis of expression of CD34, cKit, CD94, and CD56.51,52 Stage 1 cells are CD34+CD56− cells that most likely overlap with the human common lymphoid progenitor; stage 2 cells coexpress CD34 and CD117 (cKit); stage 3 cells, called immature NK cells (iNK), express CD117 and CD56; and stage 4 and 5 cells are more mature CD56+CD94+CD16− and CD56+CD94+CD16+ NK cells, respectively.
When it was found that iNK cells produced IL-22,53 it was speculated that IL-22 production was a property of iNK cells, which then lose this capacity upon maturation to conventional NK cells. However, more recent analyses have revealed that stage 3 iNK cells comprise a heterogeneous population. A substantial proportion of these cells were RORγt+, expressed CD127, and produced IL-22 and were unable to differentiate into mature NK cells in vitro, strongly suggesting that these cells within the iNK population are in fact mature ILC3s and not immature NK cells.54 Concurrently, the minority of iNK cells lacked expression of CD127, and those cells were able to differentiate into mature NK cells in vitro.54 In another study, it was confirmed that human CD56+ ILC3s generated from cord blood hematopoietic stem cells are unable to differentiate into conventional NK cells.55 In line with these observations, experiments with RORγt fate-mapped mice demonstrated that RORγt is not expressed during the development of NK cells.56,57 In addition, whereas NK cells are IL-7 independent, both human and mouse ILC3s require IL-7 for optimal development.56,57 These data clearly demonstrate that RORγt+ ILCs and conventional NK cells belong to different lineages. Because 50% of all ILC3 cells express CD56,40 CD56 cannot be used as a defining marker for NK cells, as previously thought. Other markers such as KIRs, CD94, and CD16, which are not expressed on CD127+ ILCs, should be used to distinguish between CD127+CD56+ ILC3s and conventional NK cells. LFA-1 was found to be selectively expressed in human NK cells, but not in ILC3s, qualifying LFA-1 as another marker to distinguish these 2 populations.55
With the recent identification in mice of committed precursor subsets that give rise to group 1, 2, and 3 ILCs but not to NK cells (or T and B lymphocytes), it becomes clear that NK cells indeed represent a subset of ILCs that are developmentally related to but distinct from ILC1s, ILC2s, and ILC3s.21,31 These ILC precursor subsets express the transcription factors PLZF, Id2, GATA3, and TOX and are phenotypically defined as cells that are lineage negative, IL7Rα+cKit+α4β7+. Human ILC progenitor subsets that are committed to develop into ILC1s, ILC2s, or ILC3 but have lost the potential to develop into NK cells have yet to be identified.
Tissue distribution of human ILCs
In healthy individuals, about 0.01% to 0.1% of circulating lymphocytes express a CD127+ ILC phenotype. The majority of CD127+ ILCs found in peripheral blood are group 2 ILCs,58 whereas NKp44+ ILC3s58 and CD127− ILC1s28 are nearly absent (Figure 1B). Peripheral blood ILC subsets from healthy individuals do not express cytokine transcripts, indicating that they are not activated. The composition of human ILC subsets in tissues depends on the tissue type. For instance, whereas group 2 ILCs and NKp44− ILC3s are the most prevalent ILC subsets in healthy human skin tissue,59,60 in other tissues such as thymus, tonsils, and bone marrow and in the gut, NKp44+ ILC3 is the prominent ILC subset. In the following paragraphs, we review our knowledge of human ILCs in intestine, lung, and skin. Mouse ILCs have also been identified and characterized in adipose tissue,6,61 where they promote accumulation of eosinophils and alternatively activated macrophages, which are implicated in metabolic homeostasis. Moreover, these cells are resident in the liver, and when stimulated with IL-33, they can mediate liver fibrosis.19 Thus far, there is no information available about ILCs in the human liver and adipose tissue. Recently, ILC3 were detected in the human spleen, where they interact with stromal cells for survival signals and with innate B cells to produce antibodies.62
ILCs in the intestine
In 2009, in parallel with the identification of human fetal LTi cells and postnatal tonsillar LTi-like cells,40 the first report appeared demonstrating the presence of human IL-22–producing ILC3s in the healthy gut (Figure 3A).5 These NKp44+ ILC3s, which were originally called NK22 cells, produce IL-22 that signals to epithelial cells where it promotes proliferation, IL-10 and antimicrobial peptide production, and mucus production.5 In vitro, NKp44+ ILC3s were responsive to IL-23, IL-1β, IL-2, and IL-7 by producing IL-22,43,44,63 which is enhanced in the presence of TLR263 and NKp44 ligands.64 Furthermore, human gut ILC3s express transcripts for leukemia inhibitory factor,5 which induces proliferation of epithelial cells, and IL-26,5 a cytokine that, like IL-22, belongs to the IL-10 cytokine family. IL-26 negatively modulates proliferation of intestinal epithelial cells and induces secretion of proinflammatory cytokines tumor necrosis factor (TNF) and IL-8 by these cells.65 Also, gut resident NKp44+ ILC3s produce cytokines that act on T and B cells including IL-2,63 the B-cell activating factor BAFF that supports survival and expansion of mature B cells,44 and the chemokine CCL20 that directs the migration of T and B lymphocytes and ILCs into the gut.44
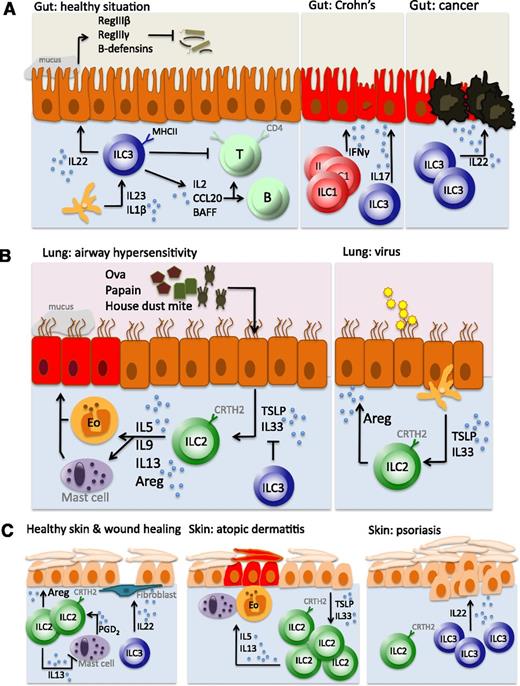
ILCs in gut, lung, and skin. (A) In the healthy situation, ILC3s produce IL-22 to maintain the epithelial barrier, generate antimicrobial products (such as RegIIIβ, RegIIIγ, and β-defensins), and suppress the reactivity of commensal bacteria-specific T cells (left panel). Crohn disease is characterized by an accumulation of IFN-γ–producing ILC1s (middle panel). During intestinal inflammation, ILC3s produce IL-22 to maintain epithelial barrier homeostasis. CRC develops when this autoregenerative function is not switched off in time (right panel). (B) Airway hypersensitivity is characterized by stromal production of TSLP and IL-33 that induces IL-5 and IL-13 production by ILC2s and subsequent recruitment and activation of eosinophils and mast cells (left panel). Upon viral airway infection, however, ILC2s are induced to produce amphiregulin, which is involved in airway epithelium repair and maintenance, and thereby function as tissue-protective cells (right panel). (C) In the healthy skin, ILC2s maintain close interactions with mast cells, suppressing their proinflammatory function, whereas ILC3s are involved in wound repair (left panel). Atopic dermatitis is an ILC2-mediated disease (middle panel), whereas ILC3s are accumulated in psoriasis (right panel).
ILCs in gut, lung, and skin. (A) In the healthy situation, ILC3s produce IL-22 to maintain the epithelial barrier, generate antimicrobial products (such as RegIIIβ, RegIIIγ, and β-defensins), and suppress the reactivity of commensal bacteria-specific T cells (left panel). Crohn disease is characterized by an accumulation of IFN-γ–producing ILC1s (middle panel). During intestinal inflammation, ILC3s produce IL-22 to maintain epithelial barrier homeostasis. CRC develops when this autoregenerative function is not switched off in time (right panel). (B) Airway hypersensitivity is characterized by stromal production of TSLP and IL-33 that induces IL-5 and IL-13 production by ILC2s and subsequent recruitment and activation of eosinophils and mast cells (left panel). Upon viral airway infection, however, ILC2s are induced to produce amphiregulin, which is involved in airway epithelium repair and maintenance, and thereby function as tissue-protective cells (right panel). (C) In the healthy skin, ILC2s maintain close interactions with mast cells, suppressing their proinflammatory function, whereas ILC3s are involved in wound repair (left panel). Atopic dermatitis is an ILC2-mediated disease (middle panel), whereas ILC3s are accumulated in psoriasis (right panel).
The observation that TLR agonists can activate NKp44+ ILC3s raised the question whether microbiota regulate not only the function but also the development of these cells. However, analysis of ILC3s in the human fetal gut revealed that the development of NKp44+ ILC3s is a programmed event independent of commensals.66 These data are consistent with mouse studies demonstrating that IL-22–producing ILC3s are present in the gut during fetal life, before intestinal colonization.12,67
Studies in mouse models showed the importance of IL-22–producing NCR+ ILC3s in the protection against colitis induced by the attaching and effacing pathogenic bacterium Citrobacter rodentium5,7,41 and in the anatomical containment of commensal intestinal bacteria.12 Recently, another role of ILC3s in the maintenance of gut homeostasis in mice was revealed. It was demonstrated that NCR− ILC3s express major histocompatibility complex (MHC) class II antigens and present microbial antigens to gut CD4+ T cells. This did not result in activation, presumably because ILC3 lack costimulatory molecules, but did result in inactivation of gut commensal bacteria–specific T-cell responses.68 Of note, human ILC3s were also found to express MHC class II molecules in this study, suggesting that this class II MHC–dependent control of T cells is also operational in the human gut. ILC3s have in addition been shown to regulate Th17 cells via the aryl hydrocarbon receptor (AHR) in mice. AHR is a ligand-dependent transcription factor; the ligands include environmental toxins such as dioxin derivatives, dietary components, and endogenous ligands such as the tryptophan metabolite. Qiu et al observed that Th17 cells were strongly increased in Ahr−/− mice.69 This effect was indirect: the absence of AHR resulted in diminished IL-22 production, which in turn caused segmented filamentous bacteria, known to stimulate Th17 cells, to expand. Whether this type of control of Th17 cells is also functional in the human gut remains to be established.
Collectively, these studies show that in mice, ILC3s play a crucial role in gut immunity by directly inducing epithelial cell proliferation, promoting epithelial-derived production of anti-inflammatory cytokines and antimicrobial peptides, preventing dissemination of commensal bacteria, and suppressing microbiota-specific proinflammatory CD4+ T-cell responses and suggest that in humans, gut ILC3s may have similar functions.
The human gut also contains CD127high ILC1s and CD127low ILC1s.28,29 CD127low ILC1s respond to danger signals originating from epithelial cells and myeloid cells, suggesting that they play a role in the immune response against pathogens that elicit these danger signals,28 but the specific pathogens that provoke a response of these cells have yet to be identified. Also, the function of human CD127high ILC1 cells has yet to be firmly established. Because there is uncertainty about the exact equivalent of these cells in mice, a precise determination of the in vivo function of CD127high ILCs is not yet possible. However, because in mice Tbet-dependent, IFN-γ–producing ILCs were found to be important in the immune response against Salmonella enterica,45 we speculate that human CD127high Tbethigh ILC1s also play a role in the immune response against pathogenic gut bacteria. This hypothesis is supported by the observation that in contrast to ILC3s and ILC2s, CD127high ILC1s are not present in the human fetal gut, suggesting that colonization with bacteria triggers the appearance of these cells.29 Interestingly, a pronounced accumulation of IFN-γ–producing CD127low ILC1s and CD127high ILC1s was observed in inflamed intestinal tissues of Crohn disease patients, whereas the frequency of NKp44+ ILC3s was diminished, possibly through IL-12–dependent differentiation of ILC3s toward ILC1.28,29 Of note, other ILC subsets such as IL-17–producing CD56− ILC3s70 are also expanded in Crohn disease patients. The importance of IL-12– and IL-23–responsive lymphocytes in the pathobiology of Crohn disease has been emphasized recently by the observation that blockade of the IL-12/IL-23 axis by ustekinumab, an inhibitor of the subunit dimer p40 that is shared between IL-12 and IL-23, led to disease remission in TNF-antagonist–resistant patients.71
The role of ILC2s in gut homeostasis and immunity has received considerably less attention. ILC2s are also present in the human gut, most prevalently in the fetal gut58 ; in adult human intestinal tissues, CRTH2+ ILC2s account for only a very small minority of the total CD127+ ILC pool. Fetal gut ILC2s express transcripts for IL-5 and IL-13 in situ, but the role of these cytokines in the fetal gut is unknown. It is possible that ILC2s play important roles in tissue generation in the gut. In the mouse, ILC2s are the source of IL-5 that regulate eosinophil homeostasis72 and IL-13 that may induce alternatively activated macrophages. Upon infection with nematodes, murine gut ILC2s contribute to clearance of worms by producing IL-973 and IL-13.32 The antihelminth and eosinophil- and alternatively activated macrophage–regulating activities of ILC2 in the human gut remain to be confirmed.
ILCs in the respiratory system
ILC2s are most thoroughly studied in the context of lung immune cell homeostasis, immunity, and inflammation (Figure 3B). Studies in the mouse have demonstrated that ILC2s are involved in the immune response against nematodes such as Nippostrogylus brasiliensis6,32,34 and in the repair of lung tissue damage inflicted by infection with pathogens through the production of the epidermal growth factor family member amphiregulin.11 CRTH2+ ST2+ ILC2s have been identified in healthy human lung,11,58 but the role of human ILC2s in lung homeostasis is currently unknown.
Inflammatory conditions of the lung are characterized by a type 2 signature. It has been recognized that type 2 cytokines are critical for pulmonary recruitment of type 2 effector cells, such as eosinophils (IL-5 and granulocyte-macrophage colony-stimulating factor), mast cells (IL-9), and immunoglobulin E–producing B cells (IL-4 and IL-13), and cytokines that directly affect target tissue (eg, IL-13–induced fibrosis). It is now recognized that not only Th2 cells but also ILC2s are the cellular source of type 2 cytokines in the lung. In humans, IL-5– and IL-13–producing innate cells that resemble ILC2s have been found in the sputum of asthma patients,74 in the lung parenchyma and bronchoalveolar lavage fluids of lung transplantation patients,11 and in patients with idiopathic pulmonary fibrosis.75 We identified CD34− CRTH2+ ILC2s in nasal polyp tissues of patients suffering from chronic rhinosinusitis (CRS), a typical type 2 inflammatory disease characterized by eosinophilia and high immunoglobulin E levels.58 Interestingly, TSLP activates human ILC2 by directly upregulating GATA3 via STAT5, resulting in the production of high amounts of type 2 cytokines.58 This observation is highly relevant in the context of CRS and also asthma, because TSLP protein expression was significantly increased in epithelial cells derived from nasal polyps of CRS patients58 and in the airway epithelium and lamina propria of asthmatic patients, particularly in patients with severe asthma.76 TSLP immunostaining in both compartments correlated with the severity of airflow obstruction. This study also provided evidence that majority of leukocytes expressing IL-13 were ILC2s.76 Taken together, these data indicate that human ILC2s are involved in lung inflammation and pathology. This conclusion is confirmed by numerous studies in mouse models of type 2 inflammatory diseases, in particular of allergic asthma15,16,77,-79 (reviewed by Walker and colleagues26,80 ). Given its potential role in inflammatory diseases of the lung, such as asthma, it is highly relevant to understand how lung-residing ILC2s are regulated in healthy humans and asthmatics. Recently it was reported that lipoxin A4, a member of a class of pro-resolving lipid mediators, decreases IL-13 production by ILC2 by interacting with ALX/FPR2 receptors.81 Lipoxin A4 also decreased numbers of eosinophils by promoting NK cell mediated apoptosis of these cells. The same study also documented that ILC2s are located in close proximity of mast cells in the lung and that the mast cell product Prostaglandin D2 (PGD2) increases IL-13 production by ILC2 through its receptor CRTH2.81 Thus the balance of lipoxin A4 and PGD2 may regulate ILC2 activity and perhaps affecting this balance by drugs may present a new therapeutic option to treat lung inflammations caused by uncontrolled ILC2 activities.
Little is known about the role of other ILC subsets in the lung. Besides ILC2s, we have also detected ILC1s and ILC3s in the human lung, but their function is unknown. In a mouse model of lung inflammation induced by ovalbumin, IL-22–producing ILC3s reduced airway inflammation by lowering the production of proinflammatory cytokines such as IL-33.82
ILCs in the skin
Several ILC subsets have been characterized in the skin of healthy wild-type mice and humans (Figure 3C). Kim et al were the first to identify an ILC subset (ILC2) in human skin, which expressed ST2, a component of the IL-33 receptor, but not CRTH2.17 The presence of ILC2 in healthy skin was confirmed by us60 and another group, but in these studies the dermal ILC2s were found to express CRTH2.83 We also detected ILC1 and NCR− ILC3s but not NCR+ ILC3s in human skin. A proportion of circulating ILC1s, CRTH2+ ILC2s, and NKp44− ILC3s expressed the skin-homing markers CLA and CCR10, suggesting that the dermal ILC populations are derived from circulating ILCs that migrate to the skin.60 In mouse skin, dermal ILC2s express CD103 and are the major cellular source of IL-13 under homeostatic conditions.18 Both human and mouse dermal ILC2s produce IL-4 when activated with IL-3384 and TSLP,18 respectively, whereas mouse lung ILC2s do not produce IL-4. It is unclear whether this reflects a differential signaling in the lung and skin or whether there is a distinct IL-4–producing ILC2 subset in the skin. Interestingly, mouse dermal ILC2s most strongly interact with mast cells, and ILC2-produced IL-13 may moderate mast cell responses.18 Whether ILC2s also interact with mast cells in human skin remains to be determined. Mast cells are able to produce prostaglandin D2 (PGD2), which is the ligand for CRTH2. PGD2 strongly enhanced production of IL-13 by human CRTH2+ ILC2s, an effect mimicked by supernatant-activated human mast cells.84 In addition, PGD2 induced migration of ILC2. Interestingly, CRTH2 antagonists strongly inhibited PGD2-mediated migration and cytokine production by ILC2.84 These data suggest an attenuating role of mast cells through its product PGD2 and raise the possibility that small molecular CRTH2 antagonists modify the function of human ILC2s in vivo.
In the diseased skin of human atopic dermatitis patients, increased numbers of ILC2s were observed compared with healthy controls,17,83 suggesting that ILC2s play a role in atopic dermatitis. Interestingly, interaction of ILC2-expressed killer lectin-like receptor G1 with E-cadherin widely expressed on keratinocytes and Langerhans cells suppressed IL-33–induced production of IL-5 and IL-13 by dermal ILC2s. This is suggestive for an involvement of ILC2s in atopic dermatitis, because interrupted E-cadherin signaling may be a key factor in the development of atopic dermatitis.83 Consistently, increased numbers of ILC2s were also found in a mouse model of atopic dermatitis. These ILC2s induced skin inflammation when stimulated with IL-2 or with the dermatitis-causing vitamin D analog calcipotriol in RAG2-deficient mice.17,18,83 In addition, transgenic overexpression of IL-33 in keratinocytes results in an atopic dermatitis-like syndrome that correlated strong infiltration of ILC2s.85
The skin also contains ILC3s, which in mice have been shown to interact with fibroblasts via the production of IL-22 to mediate wound healing.86 Dermal ILC3 may also mediate pathology. Psoriasis is an autoimmune disease of the skin that is driven by IL-17A, IL-17F, and IL-22. Topical exposure of mouse skin to the TLR7 agonist imiquod causes skin inflammation that bears some similarity to psoriasis and therefore has been used as an experimental model for psoriatic skin disease. In this model, IL-17A, IL-17F, and IL-22 contribute to disease, and these cytokines were shown to be produced by γδ-T cells and RORγt+ ILC3s.87 In patients with psoriasis, an accumulation of NCR+ ILC3s was observed in affected skin, suggesting that these IL-22–producing innate cells may be involved in the pathology of psoriasis.59,60 Interestingly, we and others have observed significantly elevated numbers of NKp44+ ILC3 not only in the diseased skin of psoriasis patients but also in the peripheral blood, whereas these cells are hardly present in the peripheral blood of healthy individuals.60,88 Interestingly, a favorable response to treatment of psoriasis with the anti-TNF antibody adalimumab in 1 patient was associated with a significant reduction of NKp44+ ILC3s in the peripheral blood.78 Future studies are needed to determine whether the number of NKp44+ ILC3s can indeed be used as a biomarker for psoriasis.
ILCs and cancer
The correlation between chronic inflammatory responses and an increased susceptibility to develop cancer has long been recognized. With the accumulating evidence that ILCs play pivotal roles in autoimmunity and inflammation as discussed in the foregoing paragraphs, it can be postulated that ILCs may also be involved in the development of malignancies. In colorectal carcinoma (CRC) patients, IL-22 was found to be highly expressed by tumor-infiltrating lymphocytes, which turned out to comprise IL-22–producing CD3+ and CD3− lymphocytes.89 Moreover, IL-22 production was significantly higher in cancerous tissue than in nontumor tissue sections of the same patients.89 Using an established mouse model of microbe-induced inflammatory bowel disease–associated CRC, it was found that IL-22–producing ILCs drove the induction and maintenance of CRC.89 Thus, CRC did not develop in mice that were depleted of IL-22–producing ILC3, and treatment with IL-22–blocking agents did protect against the development of CRC in these mice. Interestingly, neutralization of ILC3-derived IL-17 in the colon of mice did lead to a reduction in inflammation but did not prevent CRC, suggesting that the oncogenic effect of ILCs may be specifically attributable to IL-22. Another study confirmed the association of IL-22 with the occurrence of CRC in a mouse model.90 In other malignancies such as cutaneous T-cell lymphoma91 and hepatic carcinoma,92 IL-22 has been shown to play a key role in humans. In these malignancies, IL-22 is produced not only by T cells but also by non–T cells, and it will be of interest to determine whether ILC3s are that cellular source.
Hematopoietic stem cell transplantation
Considering the involvement of ILCs in inflammatory responses and tissue repair, and given the critical effects of chemotherapy, radiotherapy, and alloimmune responses on epithelial barriers and mucosal tissues, it can be postulated that ILCs are important modulators of pre- and postallogeneic hematopoietic stem cell transplantation (HSCT) immunity.
In a mouse model of acute graft-versus-host disease (GVHD), IL-22 that is produced by IL-23–responsive, radiotherapy-resistant recipient ILC3s protects intestinal stem cells against the detrimental effects of GVHD, because GVHD severity was significantly increased in the absence of ILC3s.93 The same group showed that IL-22–producing ILC3s are essential in the recovery of thymic epithelial cells after radiation-induced damage,94 suggesting that ILC3s, which should be radioresistant, may be important in post-HSCT T-lymphocyte reconstitution in these mice. We have longitudinally studied ILC recovery in a group of acute myeloid leukemia patients following induction chemotherapy and after allogeneic HSCT. Reconstituting ILCs were activated and expressed tissue-homing molecules and after allogeneic HSCT were of donor origin. Interestingly, patients with high proportions of CD69+ gut-homing ILC2s, NCR− ILC3s, and NCR+ ILCs had less mucositis and GVHD. In addition, following induction chemotherapy and after allogeneic HSCT, a large number of NKp44+ ILC3s appeared in the circulation, which was associated with an absence of GVHD.95
The post-HSCT period is characterized by a significant susceptibility to develop opportunistic infections. This is generally attributed to the absence of a full T-cell repertoire, in particular during the first 1 or 2 years after stem cell transplantation, and the frequent use of immunosuppressants including steroids, to prevent or treat GVHD. However, recent data suggest that ILCs may also be involved here, because it was shown in a mouse model that RORγt-dependent, IL-23–responsive, IL-17–producing ILCs are imperative for the clearance of fungal infections such as Candida albicans.96 In another paper, it was shown that IL-22 is important in clearance of Aspergillus fumigatus infection,97 but the source of IL-22 production in this mouse model was not specified.
Hematologic malignancies arising from ILCs or ILC progenitors
ILCs may transform into malignant cells. To identify malignancies that are derived from ILC progenitors, a more profound understanding of the developmental pathways of ILCs is needed. However, it can be speculated from data available in the literature that ILC progenitor malignancies do exist. About 4% of all acute leukemias are of ambiguous lineage (World Health Organization, 2008), including acute undifferentiated leukemias (AULs) that do not express any lineage specific antigens and mixed-phenotype leukemias coexpressing antigens of myeloid and lymphoid lineages. A proportion of the AULs are now thought to represent leukemias of NK cell progenitors. Because certain human NK cell progenitor subsets such as stage III NK cell progenitor cells recently have been shown to include ILC,53,-55 it is tempting to postulate that AULs may include ILC-lineage–derived leukemias. Several series of NK cell precursor malignancies such as myeloid/NK cell precursor acute leukemia and blastic NK cell lymphoma/leukemia have been described in the past decades. Leukemic blasts in the earliest of these series were characterized by a lymphoblastic morphology and coexpression of lymphoid markers CD7 and CD56 and myeloid markers such as CD33 and CD34.98,99 More recently, the healthy counterparts of myeloid/NK cell precursor acute leukemia were proposed to be stage 1 pro–NK cell progenitors and stage 2 pre–NK cell progenitors. In particular, stage 2 pre–NK cells are characterized by a CD34+ CD33+ CD117+ phenotype with a variable expression of CD161 and CD56100 and could therefore very well include ILCs. Precursor NK lymphoblastic lymphomas/leukemias are derived from CD34− CD33+ CD117+ CD161+ CD56+ or CD56− stage III progenitor NK cells,99 cells that include ILC3s.40,54,55,101 More extensive immunophenotyping, that includes CD161 and the IL-7 receptor CD127, in combination with analyses of transcription factor expression, is needed to further characterize these rare type leukemias and to determine to what extent they include malignantly transformed ILC progenitors.
The same holds true for difficult-to-categorize, NK cell–like lymphomas that may be derived from more mature ILC subsets. For example, refractory celiac disease is characterized by the presence of lymphocytes with an unusual phenotype that have a tendency to develop into lymphomas. It was recently observed that the nonmalignant counterparts of these aberrant lymphocytes include lineage− CD127+ CD7+ CD56+ or CD56− lymphocytes that express CD122, the IL-2/IL-15Rβ subunit, which are very similar to CD127low ILC1s described by Fuchs et al,28 and it was suggested that under the influence of chronic stimulation with IL-15, these cells may undergo malignant tranformation.102
Concluding remarks
Over the past 6 years, ILC subsets have been discovered that play important roles in innate immunity, homeostasis of a variety of cell types, and tissue (re)modeling. ILCs show a remarkable similarity with helper T-cell subsets, which has aided the rapid identification of networks of transcription factors that drive the development and function of these cells. Understandably, the knowledge of the mechanisms underlying ILC function and development in humans lags behind that of mice, but the ILC system is conserved in mice and humans, which helps in the translation of fundamental findings in ILC biology in animal model systems to that of humans. Experiments in mice have laid the groundwork for our understanding of the developmental pathways, but these pathways have yet to be fully deciphered in humans.
Studies in mouse models of inflammatory diseases indicate that ILCs can be involved in inflammatory diseases. Moreover, changes in composition of ILCs have been observed in inflamed tissues in a number of inflammatory diseases in humans. In-depth comparisons of the characteristics of ILCs in diseased tissues with those in healthy tissues will help to further delineate their possible roles in disease and to determine whether targeting ILCs will help to prevent or treat these diseases. ILCs have also been associated with cancer in mouse models. Elucidating their role in human cancer will be a challenge for the future. It seems obvious that ILCs and their precursors can undergo malignant transformation. It would be highly interesting to see whether some of the yet-undefined leukemias and lymphomas are in fact derived from (pre-)ILCs.
There is an Inside Blood Commentary on this article in this issue.
Acknowledgments
The authors would like to thank Jenny Mjösberg, Tom Cupedo, Jochem Bernink, Kristine Germar, and Marius Munneke for their critical input and Johan Dobber for technical assistance. M.D.H. is supported by The Netherlands Organization for Scientific Research (NWO-ZonMW). H.S. is supported by an advanced grant of the European Research Council.
Authorship
Contribution: H.S. and M.D.H. wrote the manuscript and designed the figures.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hergen Spits, Department of Cell Biology and Histology, Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands; e-mail: hergen.spits@amc.uva.nl.