In this issue of Blood, Wang et al describe for the first time a vital link between iron overload and pulmonary hypertension in sickle cell disease (SCD). They report this link is likely placenta growth factor (PlGF).1
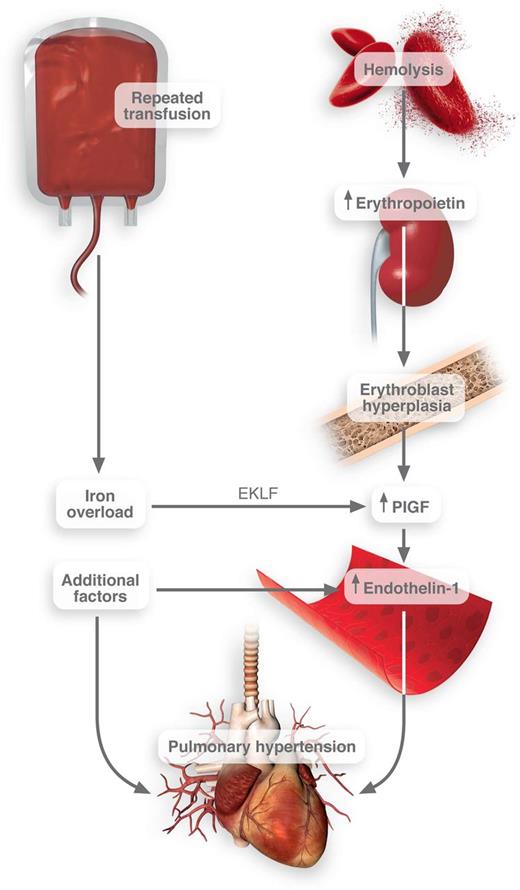
Connection between transfusion frequency and pulmonary hypertension in SCD. Iron overload induces the activation of EKLF transcription factor in erythroblasts. These cells then release PlGF, which promotes the release of endothelin-1. Thus, when combined with other pathological factors in SCD, pulmonary hypertension may result. Professional Illustration by Luk Cox.
Connection between transfusion frequency and pulmonary hypertension in SCD. Iron overload induces the activation of EKLF transcription factor in erythroblasts. These cells then release PlGF, which promotes the release of endothelin-1. Thus, when combined with other pathological factors in SCD, pulmonary hypertension may result. Professional Illustration by Luk Cox.
In the context of the profound vaso-occlusion present in SCD, the contribution of other factors to the disease (hemolysis, anemia, and transfusion dependency) can be overlooked in disease pathology. However, each of these factors likely converge to create a state of global homeostatic perturbation in which all organ systems are affected.
In fact, one of the most fundamental aspects of hemostasis, iron metabolism and equilibrium, is also disrupted. Although historically iron deficient, the increasing prevalence and rate of transfusion to (1) prevent stroke in children, (2) prepare patients for invasive surgical interventions, and (3) reduce mortality risk are tipping the balance toward the iron-overloaded patient with SCD. It is worth noting that 1 unit of red blood cells can contain up to 200 mg of iron, easily swamping a compromised, overtaxed iron sequestration system.2 The effect of iron burden on the liver is clear; the effect of iron overload on cardiac function is well documented. Wang et al, in an international effort, demonstrate that this excess iron (measured by ferritin levels) is also clearly associated with mortality in patients with SCD.1
Of note, another demonstrated risk factor for death in SCD is pulmonary hypertension. Although the prevalence of pulmonary hypertension in SCD has been the subject of some controversy, it does occur. In fact, it is 1000 to 3000 times more common in SCD than in the healthy population. Some studies report that up to 11% of patients have pulmonary hypertension.3 Furthermore, as patients with SCD survive longer, it could be predicted that the incidence of pulmonary hypertension will only increase.
Because both pulmonary hypertension and elevated iron levels are risk factors for death, Wang et al went on to ask: is there a link between excess iron and pulmonary hypertension in SCD?
Using both in vitro studies and clinical observations, they convincingly link iron with the release of PlGF. The site of action for iron in this context is the erythroblast—a population of cells typically grossly expanded in patients with SCD. They report that heme-based iron strongly induced the expression of erythroid Krüppel-like factor (EKLF), a transcription factor that regulates erythrocyte maturation.4 EKLF then promotes the transcription and release of PlGF1 (see figure).
PlGF is a member of the vascular endothelial growth factor (VEGF) family and binds primarily to VEGF receptor 1 with an affinity rivaling that of VEGF.5 Several groups have reported the up-regulation of PlGF in patients with SCD6,7 and noted the association of PlGF with echocardiography-derived tricuspid regurgitant jet velocity (TRV)—a complex measure that can suggest pulmonary hypertension. In adults, elevated TRV (>2.5 m per second) is associated with mortality.3 Wang et al also show that levels of PlGF associate with death in patients with SCD, thus underscoring the link.
This association between iron overload, PlGF, potential pulmonary hypertension, and death in SCD is perhaps not surprising. In fact, expression of PlGF in mice causes pulmonary hypertension.8 PlGF directly activates monocyte tissue factor expression and inflammatory cytokine release.7 PlGF likely up-regulates plasminogen activator inhibitor-1.9 As such, PlGF is positioned squarely in this disease to promote coagulation and deregulate fibrinolysis, each of which may promote increased pulmonary resistance. Importantly, PlGF induces the release of endothelin-1,10 one of the most powerful vasoconstrictors discovered to date. These actions could contribute to lung pathology and would squarely position iron overload as a promoter of impaired pulmonary function.
The authors went on to show the relationship between iron levels and PlGF in patients with hereditary hemochromatosis (a disorder in which the body likely absorbs excessive iron). As predicted, there was a strong relationship between ferritin levels and PlGF in these patients. In fact, as ferritin levels dropped on treatment of iron overload, PlGF levels dropped as well.
However, patients with hereditary hemochromatosis are not described as at risk for pulmonary hypertension. Does this negate the relationship between iron overload and pulmonary hypertension in SCD?
Indeed, it does not. Transfusional iron overload in SCD would present against a preexisting backdrop of ischemia/reperfusion injury, platelet activation, endothelial cell dysfunction, declining renal function, hemolysis, etc. Iron overload could be a final insult or at least be contributory in the cascade of vascular insults that start in early childhood.
Thus, although transfusion must remain a viable therapy for patients with SCD, this work provides a thought-provoking insight into the potential long-term consequences of this treatment modality.
Conflict-of-interest disclosure: The author declares no competing financial interests.