Key Points
Children with SCA and stroke show severe parenchymal and vascular abnormalities that can be assessed using a vasculopathy grading scale.
Results from the SWiTCH Trial support concerns about ineffectiveness of transfusion therapy in preventing cerebrovascular injury progression.
Abstract
The Stroke With Transfusions Changing to Hydroxyurea (SWiTCH) trial compared standard (transfusions/chelation) to alternative (hydroxyurea/phlebotomy) treatment to prevent recurrent stroke and manage iron overload in children chronically transfused over 7 years before enrollment. Standardized brain magnetic resonance imaging/magnetic resonance angiography (MRA) and transcranial Doppler (TCD) exams were performed at entry and exit, with a central blinded review. A novel MRA vasculopathy grading scale demonstrated frequent severe baseline left/right vessel stenosis (53%/41% ≥Grade 4); 31% had no vessel stenosis on either side. Baseline parenchymal injury was prevalent (85%/79% subcortical, 53%/37% cortical, 50%/35% subcortical and cortical). Most children had low or uninterpretable baseline middle cerebral artery TCD velocities, which were associated with worse stenoses (incidence risk ratio [IRR] = 5.1, P ≤ .0001 and IRR = 4.1, P < .0001) than normal velocities; only 2% to 12% had any conditional/abnormal velocity. Patients with adjudicated stroke (7) and transient ischemic attacks (19 in 11 standard/8 alternative arm subjects) had substantial parenchymal injury/vessel stenosis. At exit, 1 child (alternative arm) had a new silent infarct, and another had worse stenosis. SWiTCH neuroimaging data document severe parenchymal and vascular abnormalities in children with SCA and stroke and support concerns about chronic transfusions lacking effectiveness for preventing progressive cerebrovascular injury. The novel SWiTCH vasculopathy grading scale warrants validation testing and consideration for use in future clinical trials. This trial was registered at www.clinicaltrials.gov as #NCT00122980.
Introduction
Overt stroke is among the most serious complications to occur in young patients with sickle cell anemia (SCA), affecting 5% to 10% of children, with a peak incidence in the first decade of life.1,2 By conventional magnetic resonance imaging (MRI), even infants with SCA demonstrate brain parenchymal abnormalities,3 and intracranial damage worsens over time.4,5 Stroke is a major cause of morbidity1,6 and mortality5,7 in SCA, and current efforts seek to prevent both primary (preventive) and secondary (recurrent) stroke in this high-risk population.
The historical stroke recurrence rate of 50% to 90% for untreated children with SCA1-3 is reduced to 10% to 23% with chronic erythrocyte transfusion therapy,8,9 aiming to maintain levels of sickle hemoglobin (HbS) <30%. Although never tested formally in a randomized clinical trial, chronic transfusions are standard treatment of secondary stroke prevention in SCA, and even after a prolonged period of transfusion prophylaxis, stroke recurrence remains high after transfusions are stopped.10 Unfortunately, chronic transfusion therapy is associated with serious morbidities such as erythrocyte allo- and auto-antibody formation,11 risk of blood-borne infections,11,12 and iron overload requiring chelation therapy and frequent monitoring.12 Accumulating data suggest that transfusion-acquired iron overload is associated with significant organ damage in this patient population.5,13,14
Hydroxyurea has proven laboratory and clinical benefits for the treatment of children with SCA and can decrease the frequency of vaso-occlusive events and other acute complications.7,10,15-19 Single-institution pilot data suggested that hydroxyurea offers a safe alternative to transfusions for the prevention of recurrent stroke in children with SCA, with a recurrent stroke rate similar to that of transfusion therapy but improved iron management, with monthly phlebotomy replacing transfusion and chelation therapy in those treated with hydroxyurea.20,21 Subsequently, a phase 3 multi-institutional randomized clinical trial, Stroke With Transfusions Changing to Hydroxyurea (SWiTCH), was sponsored by the National Heart, Lung, and Blood Institute. SWiTCH enrolled pediatric subjects with a documented prior clinical stroke who were currently receiving chronic blood transfusions for secondary stroke prevention.22 The study design compared 30 months of alternative therapy (hydroxyurea/phlebotomy) to standard therapy (transfusions/chelation) for secondary stroke prevention and better management of transfusion-related iron overload. SWiTCH was closed early by the National Heart, Lung, and Blood Institute due to statistical futility for the primary composite end point, with no advantage in liver iron concentration to offset the predicted higher incidence of recurrent stroke in the alternative treatment arm.23 On the basis of these results, transfusions and chelation remain a safer way to manage children with SCA and a history of clinical stroke; however, the short duration of phlebotomy may have precluded a favorable impact on iron loading that has been demonstrated over a longer time frame.24
Because infarction and clinical stroke in SCA often result from preexisting cerebrovascular disease, careful evaluation of the intracranial vasculature is important for understanding the etiology and optimal management of stroke in this patient population. Accordingly, all SWiTCH subjects underwent standardized conventional MRI, transcranial Doppler (TCD), and magnetic resonance angiography (MRA) of the brain and cerebral vessels at study baseline (entry) and completion (exit) evaluations. These comprise a unique set of modern diagnostic imaging data, the largest and most in-depth analysis of a cohort with advanced severe cerebrovascular disease in SCA reported to date. We hypothesized that cerebrovascular disease by MRA and TCD would correlate with MRI abnormalities; in this report, we identified several correlations and developed a MRA vasculopathy grading scale, which may have prognostic significance for recurrent stroke. This novel vasculopathy grading scale warrants further validation studies and consideration for use in future prospective clinical trials involving patients with SCA and cerebrovascular disease.
Methods
Study design and subject enrollment
Details about the SWiTCH trial design and population are published.22,23 The study was approved by the local Institutional Review Board of each clinical site. Informed consent and assent (children 7-17 years of age) were obtained prior to participation. The study was conducted in accordance with the Declaration of Helsinki. The study inclusion criteria included: patients with SCA, age range 5.0-18.9 years at enrollment; previous completed overt clinical (index) stroke after age 1 year with central confirmation; ≥18 months of erythrocyte transfusions since the index stroke; transfusional iron overload with liver iron concentration ≥5.0 mg of iron/gram of dry weight liver or serum ferritin ≥500 ng/mL on 2 independent measurements; and adequate monthly erythrocyte transfusions with average HbS ≤45% for the past 6 months before enrollment.25 Recognizing that the use of aspirin for secondary stroke prophylaxis varied among academic centers, the SWiTCH protocol neither recommended nor prohibited its use. A total of 161 subjects enrolled in the SWiTCH trial; 150 and 146 with entry brain MRI/MRA and TCD examinations during the initial screening period, respectively.
MRI and MRA examinations
Standardized brain MRI/ MRA imaging was required during screening and at study exit after 30 months of treatment. Centers were allowed to use a variety of 1.5-T closed magnet MRI equipment vendors. A standardized protocol developed by the SWiTCH Neuroradiology Consultant (supplemental Table 1, available on the Blood Web site) required noncontrasted sagittal T1, axial T1, fluid-attenuated inversion recovery (FLAIR), and T2; additionally, diffusion-weighted images with an apparent diffusion coefficient map increased the sensitivity for detection of acute ischemia. The MRA images used a 3-dimensional time of flight technique with a short echo time and included both source and maximum intensity projection reformatted images in the anterior to posterior and right to left projections to assess the patency of the intracerebral arterial vessels. All MRI/MRA studies were masked to demographic and clinical information and centrally reviewed by a single reviewer (K.J.H.) blinded to clinical site, treatment arm, and neurological status.
Standardized scoring of intraparenchymal brain MRI abnormalities was performed, recording the presence, size, location, and vascular territory of each region of subcortical (leukoencephalopathy/lacunae) and/or cortical infarcts. Leukoencephalopathy was defined as white matter hyperintensities observed on FLAIR sequences, ranging from small (<5 mm), medium (5-15 mm), to large (>15 mm). Lacunae were defined as small (<5 mm) regions of punctate hypointensity on FLAIR. A cortical infarct was defined as a larger (>1.5 cm) area of encephalomalacia, involving either branch arterial or very large borderzone territories. Transient ischemic attack (TIA) in SWiTCH was defined as a brief episode of neurological dysfunction caused by focal brain or retinal ischemia, with clinical symptoms typically lasting <1 hour and no evidence of acute infarction or restricted diffusion-weighted imaging abnormalities.
Brain MRA examinations were reviewed for vessel stenoses in both the anterior and posterior circulations. A standardized scale for vessel stenosis was developed during initial reviews to provide a means of accurately assessing the location, extent, and severity of vascular disease (Figure 1). This vasculopathy scale was then used prospectively for all baseline and exit SWiTCH brain MRA examinations. Four segments of each internal carotid artery (ICA), and 3 segments of each anterior cerebral artery (ACA) and middle cerebral artery (MCA) were examined, with results recorded for a total of 20 vessel segments per subject (10 per cerebral hemisphere). Vessel stenosis was recorded by vascular location, frequency (number of stenoses), length (millimeters), and severity (percentage vessel occlusion). Severity of vessel segment occlusion was graded as mild (25-49%), moderate (50-74%), severe (75-99%), or occlusion (>99%).
Grading of vascular occlusion based on MRA. Each cerebral hemisphere and its corresponding vessels were reported separately because they represent functionally and structurally independent systems. Vessels occlusions of ≤5 mm in length are not counted. Stenosis is scored as mild (25-49%), moderate (50-74%), severe (75-99%), or occlusion (>99%).
Grading of vascular occlusion based on MRA. Each cerebral hemisphere and its corresponding vessels were reported separately because they represent functionally and structurally independent systems. Vessels occlusions of ≤5 mm in length are not counted. Stenosis is scored as mild (25-49%), moderate (50-74%), severe (75-99%), or occlusion (>99%).
TCD measurements
Local TCD equipment and examiners were used; each examiner was certified by the Neurology and TCD Core after submitting sample exams for central review. Either TCD or Transcranial Doppler imaging could be used, and cutoff values were adjusted accordingly. Standardized procedures were developed by the SWiTCH Neurology Consultant (supplemental Table 2). TCD examinations were attempted on each SWiTCH subject before study treatment initiation and prior to a scheduled transfusion. Regions of special interest included the ACA, distal ICA (DICA), bifurcation of the ICA (BIF), first segment MCA (M1), MCA, and posterior cerebral artery (PCA). TCD results were scored centrally by a blinded reviewer (R.J.A.) and subsequently classified into 1 of 4 categories for each vessel: conditional/abnormal (≥170 cm/s), ptnormal (70-169 cm/s), low (<70 cm/s for all vessels except the PCA or DICA, where low was defined as <50 cm/s), and uninterpretable if the TCD velocity was not interpretable on measurement or was missing. Categories of abnormal and conditional velocity groups were later combined to a category of conditional/abnormal due to limited numbers and previously reported significant association with stroke.26
Clinical variables
Baseline hematology measurements were performed at the randomization visit and just before a transfusion, including hemoglobin concentration, hematocrit, fetal hemoglobin, absolute reticulocyte count, white blood cell count, absolute neutrophil count, serum ferritin, aspartate aminotransferase, total bilirubin, and lactate dehydrogenase. Additional analyses with liver iron concentration, as well as genetic determination of Glucose-6-phosphate dehydrogenase deficiency deficiency or α-thalassemia trait, were compared with the MRA Vasculopathy Scale.
Statistical analysis
All demographic, MRA, MRI, and TCD variables were summarized by time point and hemisphere, where appropriate. McNemar’s test or Bowker’s test, its multigroup extension, was used to compare the distribution of MRI abnormalities and the MRA severity score between left and right hemispheres. The relationship between MRI abnormality and MRA severity scale was tested using a χ2 test. For clinical or laboratory measures, an analysis of variance modeled the relationships with the SWiTCH MRA vasculopathy scale and logistic regression modeled the relationships with presence of MRI abnormalities. A Poisson model was used to model the relationship between number of stenoses and TCD category, controlling for age at index stroke, history of TIA, and hemisphere. No adjustments were made for multiple comparisons.
Results
Subject characteristics
The mean age of the subjects was 13.1 ± 3.9 years (range, 5.0-19 years) at study enrollment; 51% were boys. Their mean age at index stroke was 5.9 ± 2.8 years (range, 1.1-14.8 years); the average length of time between the index stroke and SWiTCH baseline studies was >7 years. At study entry, 10% of the subjects had a history of recurrent stroke on transfusion therapy, 15% had a history of TIAs, 25% had a history of either, and 2% had a history of both. A total of 150 baseline and 112 exit brain MRI/MRA examinations were completed and centrally evaluated. There were 38 subjects without an exit brain MRI/MRA examination due to failure to reach randomization (10), subject refusal or nonadherence (9), positive on-study stroke adjudication (7), withdrawal (6), death (1), or various other reasons (5). A total of 146 baseline TCD examinations were performed; 98 had interpretable velocities on the left hemisphere and 105 on the right hemisphere. At study exit, 111 TCD examinations were performed; 69 were interpretable on the left side and 71 were interpretable on the right side. For all subjects, the median duration between baseline and exit studies was 26 months (interquartile range IQR, 17-31 months).
MRI and MRA findings
SWiTCH subjects had extensive intracranial parenchymal damage noted on baseline examinations, consistent with their history of previous clinical stroke (Table 1). Specifically, 88% of subjects showed some form of parenchymal abnormality in the left hemisphere and 81% in the right hemisphere, with 71% showing bilateral abnormalities. Each cerebral hemisphere and its corresponding vessels were reported separately because they represent functionally and structurally independent systems.27 A total of 85% of subjects had subcortical abnormalities identified on the left side and 79% on the right side, with 68% having bilateral findings. Cortical infarcts were observed in 53% of subjects on the left and 37% on the right, with 23% being bilateral. There were 50% of subjects with both subcortical and cortical infarcts noted in the left hemisphere and 32% with both types on the right side (Table 1). More MRI abnormalities were observed in the left hemisphere, and these differences were significant both for cortical (P = .004) and combined subcortical/cortical infarcts (P = .005).
SWiTCH brain MRI and MRA findings by hemisphere and study time point
| . | Baseline (N = 150) . | Exit (N = 112) . | ||||
|---|---|---|---|---|---|---|
| Left . | Right . | Bilateral . | Left . | Right . | Bilateral . | |
| MRI | ||||||
| Location of lesion | ||||||
| Any abnormality | 132 (88%) | 122 (81%) | 106 (71%) | 101 (90%) | 92 (82%) | 82 (73%) |
| Subcortical infarct | 127 (85%) | 118 (79%) | 102 (68%) | 96 (86%) | 88 (79%) | 78 (70%) |
| Cortical infarct* | 80 (53%) | 56 (37%) | 34 (23%) | 59 (53%) | 44 (39%) | 27 (24%) |
| Subcortical and cortical* | 75 (50%) | 52 (35%) | 30 (20%) | 54 (48%) | 40 (36%) | 23 (21%) |
| MRA | ||||||
| Number of stenoses | ||||||
| 0 | 63 (42%) | 81 (54%) | 50 (45%) | 62 (55%) | ||
| 1-2 | 13 (9%) | 13 (9%) | 8 (7%) | 9 (8%) | ||
| 3-4 | 33 (22%) | 20 (13%) | 23 (21%) | 13 (12%) | ||
| ≥5 | 41 (27%) | 36 (24%) | 31 (28%) | 28 (25%) | ||
| Mean number of stenoses per subject | 2.8 | 2.3 | 2.8 | 2.3 | ||
| Subjects with stenosis | 87 (58%) | 69 (46%) | 52 (35%) | 62 (55%) | 50 (45%) | 40 (36%) |
| Maximum stenosis length (mm) | ||||||
| 6-15 | 16 (11%) | 11 (7%) | 11 (10%) | 11 (10%) | ||
| 16-25 | 34 (23%) | 28 (19%) | 24 (21%) | 18 (16%) | ||
| ≥26 | 37 (25%) | 28 (19%) | 27 (24%) | 21 (19%) | ||
| Occlusion grade | ||||||
| Grade 0 | 63 (42%) | 81 (54%) | 50 (45%) | 62 (55%) | ||
| Grade 1-3 | 8 (5%) | 8 (5%) | 8 (7%) | 7 (6%) | ||
| Grade 4-6 | 79 (53%) | 61 (41%) | 54 (48%) | 43 (38%) | ||
| . | Baseline (N = 150) . | Exit (N = 112) . | ||||
|---|---|---|---|---|---|---|
| Left . | Right . | Bilateral . | Left . | Right . | Bilateral . | |
| MRI | ||||||
| Location of lesion | ||||||
| Any abnormality | 132 (88%) | 122 (81%) | 106 (71%) | 101 (90%) | 92 (82%) | 82 (73%) |
| Subcortical infarct | 127 (85%) | 118 (79%) | 102 (68%) | 96 (86%) | 88 (79%) | 78 (70%) |
| Cortical infarct* | 80 (53%) | 56 (37%) | 34 (23%) | 59 (53%) | 44 (39%) | 27 (24%) |
| Subcortical and cortical* | 75 (50%) | 52 (35%) | 30 (20%) | 54 (48%) | 40 (36%) | 23 (21%) |
| MRA | ||||||
| Number of stenoses | ||||||
| 0 | 63 (42%) | 81 (54%) | 50 (45%) | 62 (55%) | ||
| 1-2 | 13 (9%) | 13 (9%) | 8 (7%) | 9 (8%) | ||
| 3-4 | 33 (22%) | 20 (13%) | 23 (21%) | 13 (12%) | ||
| ≥5 | 41 (27%) | 36 (24%) | 31 (28%) | 28 (25%) | ||
| Mean number of stenoses per subject | 2.8 | 2.3 | 2.8 | 2.3 | ||
| Subjects with stenosis | 87 (58%) | 69 (46%) | 52 (35%) | 62 (55%) | 50 (45%) | 40 (36%) |
| Maximum stenosis length (mm) | ||||||
| 6-15 | 16 (11%) | 11 (7%) | 11 (10%) | 11 (10%) | ||
| 16-25 | 34 (23%) | 28 (19%) | 24 (21%) | 18 (16%) | ||
| ≥26 | 37 (25%) | 28 (19%) | 27 (24%) | 21 (19%) | ||
| Occlusion grade | ||||||
| Grade 0 | 63 (42%) | 81 (54%) | 50 (45%) | 62 (55%) | ||
| Grade 1-3 | 8 (5%) | 8 (5%) | 8 (7%) | 7 (6%) | ||
| Grade 4-6 | 79 (53%) | 61 (41%) | 54 (48%) | 43 (38%) | ||
Each cerebral hemisphere and its corresponding vessels were reported separately because they represent functionally and structurally independent systems.
P < .05 using a McNemar’s test to compare distribution between hemispheres.
Substantial vascular damage was also present on baseline brain MRA examinations, as measured by the number of vessels involved, severity of stenosis, and length of stenosis (Table 1). A total of 58% of subjects had left stenosis, and 46% had right vessel stenosis. The mean number of stenotic segments per subject was 2.8 on the left and 2.3 on the right; most exceeded 15 mm in length. This severity was also evidenced by the grading scale score: on the left hemisphere, 53% had at least grade 4 stenosis; on the right, 41% had at least grade 4 stenosis. However, some subjects had no baseline vessel stenosis: 42% had a 0 score on the left hemisphere and 54% on the right hemisphere. The left hemisphere consistently showed more stenosis than the right hemisphere, although it did not reach statistical significance as measured by the MRA grading scale (P = .07). The presence of moya-moya vasculopathy was associated with severe vasculopathy; however, visualization of these tiny vessels was inconsistent across all 25 institutions on a 1.5-T magnet and were not included in the vasculopathy grading scale. Fifteen children had left hemisphere (4 = grade 5, 11 = grade 6) and 13 children also had right hemisphere (1 = grade 5, 12 = grade 6) moya-moya vasculopathy.
Study exit examinations were performed in 112 children (75%) who did not experience an adjudicated stroke and the examinations revealed few changes. Only 1 subject (alternative treatment) developed a new subcortical lacuna, consistent with silent infarction occurring during the study treatment period. Similarly, only 1 subject had progressive vasculopathy (evolved from grade 0 to grade 4); this subject was also in the alternative treatment arm.
Positive stroke adjudication evaluations occurred in 7 SWiTCH subjects as previously described (Table 2).23 These children had severe parenchymal disease at study enrollment, including subcortical infarcts (left and right hemispheres: 6 children each), cortical infarcts (4 on the left, 3 on the right), and both subcortical/ cortical infarcts (4 and 3, respectively). Seven had evidence of severe cerebrovascular disease, with 86% of hemispheres having a vasculopathy score of grade 4 or higher. One subject had a baseline vasculopathy score of 0 on the right, which progressed to 5 at the time of an ipsilateral cortical/subcortical infarct.
Details of stroke events, including vasculopathy grade, for subjects who experienced stroke during the study
| Subject . | Baseline grade right . | Baseline grade left . | Event grade right . | Event grade left . | Infarct size . | Infarct location . | Cortical . | Subcortical . | Both . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 6 | 5 | 6 | Medium | Left | No | Yes | No |
| 2 | 0 | 6 | 5 | 6 | Large | Right | Yes | No | No |
| 3 | 4 | 5 | 4 | 5 | Small | Right | No | Yes | No |
| 4 | 6 | 6 | 6 | 6 | Small | Right | No | Yes | No |
| 5 | 5 | 5 | 5 | 5 | Medium | Left | Yes | No | No |
| 6* | 6 | 5 | * | * | Large, hemorrhagic | Right > bilateral | Yes | Yes | Yes |
| 7 | 5 | 0 | 5 | 0 | Small | Bilateral | No | Yes | No |
| Subject . | Baseline grade right . | Baseline grade left . | Event grade right . | Event grade left . | Infarct size . | Infarct location . | Cortical . | Subcortical . | Both . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 6 | 5 | 6 | Medium | Left | No | Yes | No |
| 2 | 0 | 6 | 5 | 6 | Large | Right | Yes | No | No |
| 3 | 4 | 5 | 4 | 5 | Small | Right | No | Yes | No |
| 4 | 6 | 6 | 6 | 6 | Small | Right | No | Yes | No |
| 5 | 5 | 5 | 5 | 5 | Medium | Left | Yes | No | No |
| 6* | 6 | 5 | * | * | Large, hemorrhagic | Right > bilateral | Yes | Yes | Yes |
| 7 | 5 | 0 | 5 | 0 | Small | Bilateral | No | Yes | No |
Subject 6 had right thalamic hemorrhagic stroke (near a small baseline MRA moya-moya vessel), with associated diffuse subarachnoid hemorrhage, and right → left ischemic infarcts from associated vasospasm. The subject presented in extremis, and only a CT was performed.
Nineteen stroke adjudication evaluations in 17 subjects were negative for clinical stroke but positive for TIA; these 19 events occurred in both treatment arms (standard treatment arm, 11; alternative treatment arm, 8). Subjects who had a TIA during the study generally had milder vasculopathy than those with recurrent stroke, but no relationship was identified between baseline or exit MRI or MRA vasculopathy findings and development of TIA.
TCD findings
All SWiTCH subjects had a TCD examination attempted at study entry, but the majority of children had low or uninterpretable baseline velocities detectable in the left (87%) and right (83%) hemispheres and 76% bilaterally. By region in the left hemisphere, a total of 69% ACA, 52% BIF, 48% DICA, 68% M1, 44% MCA, and 55% PCA evaluations were either low or uninterpretable (Figure 2). In the right hemisphere, a total of 59% ACA, 50% BIF, 49% DICA, 61% M1, 39% MCA, and 54% PCA evaluations were either low or uninterpretable. In contrast, very few SWiTCH subjects had conditional/abnormal TCD velocities. Normal TCD velocities were identified in less than one-half of vessels at study entry. Results were similar among the 112 subjects with a recorded TCD velocity at both entry and at exit: 88% of the left-sided vessels and 87% of right-sided vessels were low or uninterpretable. There was no discernible pattern of TCD change by category or direction based on study treatment.
SWiTCH TCD velocity results by cerebral hemisphere and study time point.
Relationships among brain MRI, MRA, and TCD measurements
A higher proportion of subjects with more severe stenosis had abnormal MRI findings. Among subjects with no detectable vasculopathy (score 0), 37% had cortical infarcts in the left hemisphere and 23% in the right hemisphere. Among subjects with severe vasculopathy (score 4-6), 66% had cortical infarcts on the left and 56% on the right (P = .002 and P = .0004, respectively). Similar distributions were observed for subcortical abnormalities (P = .003, left; P = .3203, right), as well as the combination of subcortical abnormalities plus cortical infarcts (P = .0007, left; P = .002, right; Table 3). Similar findings were observed at study exit. There was no association between size of infarct and vasculopathy grade.
SWiTCH brain MRI results by MRA vessel occlusion grading score
| . | MRA severity grade . | |||||
|---|---|---|---|---|---|---|
| Left hemisphere . | Right hemisphere . | |||||
| 0 . | 1-3 . | 4-6 . | 0 . | 1-3 . | 4-6 . | |
| Baseline | ||||||
| No. of subjects | 63 | 8 | 79 | 81 | 8 | 61 |
| Subcortical | 46 (73%) | 7 (88%) | 74 (94%)* | 60 (74%) | 7 (88%) | 51 (84%) |
| Cortical Infarct | 23 (37%) | 5 (63%) | 52 (66%)* | 19 (23%) | 3 (38%) | 34 (56%)* |
| Cortical and subcortical | 20 (32%) | 5 (63%) | 50 (63%)* | 18 (22%) | 3 (38%) | 31(51%)* |
| On study | ||||||
| Stroke | 1 (2%) | 0 | 2 (3%) | 1 (1%) | 0 | 3 (3%) |
| End of study | ||||||
| No. of subjects | 50 | 8 | 54 | 62 | 7 | 43 |
| Subcortical | 39 (78%) | 7 (88%) | 50 (93%) | 45 (73%) | 7 (100%) | 36 (84%) |
| Cortical Infarct | 21 (42%) | 4 (50%) | 34 (63%) | 17 (27%) | 3 (43%) | 24 (56%)* |
| Cortical and subcortical | 18 (36%) | 4 (50%) | 32 (59%) | 16 (26%) | 3 (43%) | 21 (49%)* |
| . | MRA severity grade . | |||||
|---|---|---|---|---|---|---|
| Left hemisphere . | Right hemisphere . | |||||
| 0 . | 1-3 . | 4-6 . | 0 . | 1-3 . | 4-6 . | |
| Baseline | ||||||
| No. of subjects | 63 | 8 | 79 | 81 | 8 | 61 |
| Subcortical | 46 (73%) | 7 (88%) | 74 (94%)* | 60 (74%) | 7 (88%) | 51 (84%) |
| Cortical Infarct | 23 (37%) | 5 (63%) | 52 (66%)* | 19 (23%) | 3 (38%) | 34 (56%)* |
| Cortical and subcortical | 20 (32%) | 5 (63%) | 50 (63%)* | 18 (22%) | 3 (38%) | 31(51%)* |
| On study | ||||||
| Stroke | 1 (2%) | 0 | 2 (3%) | 1 (1%) | 0 | 3 (3%) |
| End of study | ||||||
| No. of subjects | 50 | 8 | 54 | 62 | 7 | 43 |
| Subcortical | 39 (78%) | 7 (88%) | 50 (93%) | 45 (73%) | 7 (100%) | 36 (84%) |
| Cortical Infarct | 21 (42%) | 4 (50%) | 34 (63%) | 17 (27%) | 3 (43%) | 24 (56%)* |
| Cortical and subcortical | 18 (36%) | 4 (50%) | 32 (59%) | 16 (26%) | 3 (43%) | 21 (49%)* |
Percentages represent the proportion of subjects with an MRI lesion for a given MRA vasculopathy grade range.
P < .05 using the χ2 test.
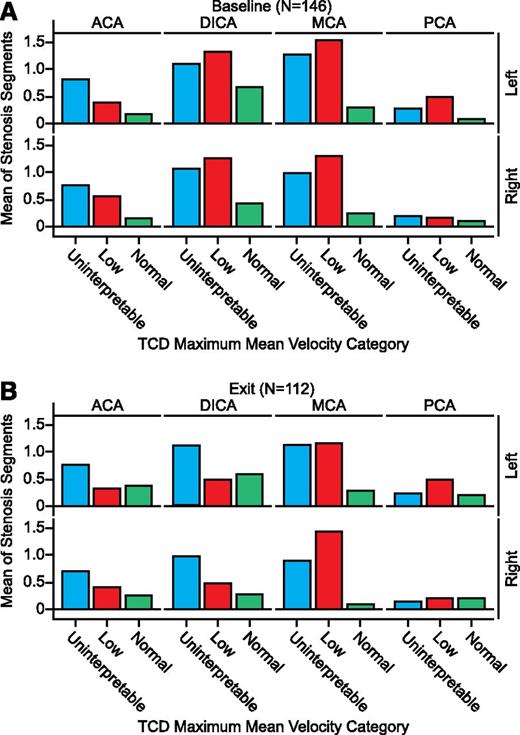
Across the ACA, DICA, MCA, and PCA vascular regions, subjects with low or uninterpretable TCD velocities had a higher mean number of stenoses (Figure 2) and stenotic segments (Figure 3) than those with normal TCD velocities. Adjusting for age at index stroke, hemisphere, and history of TIA, SWiTCH subjects with low or uninterpretable TCD velocities had significantly higher rates of stenoses in the MCA region (incidence risk ratio [IRR] = 5.1, P≤.0001 and IRR = 4.1, P < .0001, respectively; Table 4). Similar findings were noted within the DICA (IRR = 1.9 low, P = .006; IRR = 1.9 uninterpretable, P < .0001). The uninterpretable category in the ACA region (IRR = 1.3, P = .004) and low category in PCA (IRR = 2.8. P = .031) also were significant.
Segmental stenosis in SWiTCH subjects by TCD velocity, vessel region, and hemisphere. (A) Baseline studies. (B) Exit studies.
Segmental stenosis in SWiTCH subjects by TCD velocity, vessel region, and hemisphere. (A) Baseline studies. (B) Exit studies.
IRR for vessel stenosis in SWiTCH subjects with either low or uninterpretable TCD velocities compared with normal TCD velocities
| Vasculopathy region . | TCD result . | IRR (95% CI) . | P value . |
|---|---|---|---|
| ACA | Low | 1.2 (1.0, 1.5) | .073 |
| Uninterpretable | 1.3 (1.1, 1.6) | .004 | |
| DICA | Low | 1.9 (1.2, 3.1) | .006 |
| Uninterpretable | 1.9 (1.4, 2.5) | <.0001 | |
| MCA | Low | 5.1 (3.1, 8.3) | <.0001 |
| Uninterpretable | 4.1 (2.7, 6.4) | <.0001 | |
| PCA | Low | 2.8 (1.1, 6.9) | .031 |
| Uninterpretable | 1.8 (0.7, 4.6) | .227 |
| Vasculopathy region . | TCD result . | IRR (95% CI) . | P value . |
|---|---|---|---|
| ACA | Low | 1.2 (1.0, 1.5) | .073 |
| Uninterpretable | 1.3 (1.1, 1.6) | .004 | |
| DICA | Low | 1.9 (1.2, 3.1) | .006 |
| Uninterpretable | 1.9 (1.4, 2.5) | <.0001 | |
| MCA | Low | 5.1 (3.1, 8.3) | <.0001 |
| Uninterpretable | 4.1 (2.7, 6.4) | <.0001 | |
| PCA | Low | 2.8 (1.1, 6.9) | .031 |
| Uninterpretable | 1.8 (0.7, 4.6) | .227 |
Risk ratios were determined using a Poisson model controlling for age at index stroke, previous history of TIA, and hemisphere.
No significant associations were identified between the severity of vasculopathy by MRA score or presence of MRI abnormalities and baseline hematological measurements performed during randomization and just before transfusion (data not shown). Bilirubin was associated with increased severity of MRA in the left hemisphere (P = .04), but the α-thalassemia trait showed no relationship to MRA severity. Among boys, the presence of G6PD deficiency (A− variant) was associated with milder (grades 1-3) vasculopathy in the right hemisphere compared with more severe vasculopathy in boys without G6PD deficiency (P = .0005).
Similarly, we investigated the relationship between vasculopathy and gender, age at index stroke, age at enrollment, and duration of transfusion. The duration of transfusion (P = .043) and age at index stroke (P = .007) were positively associated severity of vasculopathy in the right hemisphere. For brain MRI abnormalities, only age at index stroke showed an association with the presence of cortical infarct in the left hemisphere (P = .01), whereas only white blood cell count showed an association with the presence of subcortical infarct in the left hemisphere (P = .01).
Discussion
Stroke is arguably the most devastating complication to occur in children with SCA, because it causes severe morbidity, generally with both motor and cognitive impairment2,16,28-31 ; recurrence and progression are common unless lifelong transfusions or stem cell transplantation is provided. Symptomatic infarction is usually associated with abnormalities of large intracranial arteries,16,32-34 with the most frequent site of vascular involvement being the distal ICA and proximal portions of the MCA and ACAs.35,36 Histological examination of stenotic arterial lesions shows proliferation of fibrous tissue and fragmentation of the internal elastic lamina.37 Incremental thrombus formation,38 possibly with incorporated sickled red blood cells,37 has been suggested as a cause of vascular lesions. Direct injury to the endothelium is probably involved,16 and abnormal adherence of erythrocytes, elevated leukocytes, and other circulating blood cells may also play a role.39 Infarction results from occlusion of major feeding arteries and severe reduction of regional cerebral perfusion.
Although TCD is recognized as a sensitive predictor of stroke risk, this screening technique is relatively nonspecific and provides limited anatomical information regarding the cerebral vasculature. MRI and MRA are the preferred techniques to evaluate brain parenchymal and vessel disease. The current study provides the results of prospective evaluation using all 3 modalities on a well-defined cohort of children with SCA and documented stroke and thus represents the largest and most definitive dataset reported to date. One of the main strengths of this comparative analysis is that standardized methodologies were used for collecting MRI, MRA, and TCD data in a multicenter fashion. Detailed instructions were provided to each clinical site, and, despite differences in MRI equipment vendors, the images were equivalent and allowed full ascertainment of both parenchymal and vascular disease. In addition, all brain MRI/MRA and TCD exams were read by separate, blinded central reviewers (K.J.H. and R.J.A., respectively). Extensive disease was identified using all 3 modalities, but absence of vessel flow on most TCD exams reduces its utility for assessment and management of children who already had a clinically overt stroke.
The development of a standardized vasculopathy grading scale that is easily implemented for the cerebrovasculature is a novel and important outcome of this investigation and potentially a scale that can be used in future clinical trials. The comprehensive MRA-based vasculopathy grading system (Figure 1) defined the stenosis location, number of affected segments, length of the stenosis, and severity of vessel occlusion, without an a priori expectation of the findings; the easily implementable scoring system provides a stepwise scale to document progressive vascular disease. Considering that the MRA review included the assessment of 4 segments of the ICA, as well as 3 segments of the ACA and MCA, respectively, thousands of vessel segments were assessed both at baseline and in end-of-study examinations. Future clinical trials involving cerebrovascular disease in SCA can now use this vasculopathy grading scale; the ongoing TCD With Transfusions Changing to Hydroxyurea (TWiTCH, #NCT00112900) is currently using the same scale for central readings of brain MRI/MRA examinations.
Many children enrolled in SWiTCH had evidence of severe vasculopathy on MRA, which is congruent with the clinical phenotype of stroke, but a surprising number (left hemisphere = 42%, right hemisphere = 54%) had no evidence of severe vessel stenosis, with vasculopathy scores of 0. For those with minimal or absent vasculopathy, their original stroke presumably did not result from acute occlusion within the Circle of Willis, but may have been associated with an acute “environmental event” such as anemia, hypotension, infection, or dehydration. However, they had very similar patterns of parenchymal brain injury as those with severe vasculopathy. Children with abnormal MRI/MRA findings typically had widespread and extensive disease—multiple types of parenchymal disease involving both cortical and subcortical regions, as well as diffuse and bilateral vascular stenosis. Possibly these children have a heritable predisposition toward inflammation or other pathways that contribute to cerebrovascular disease, which thus serve as a risk factor for their strokes.
Unexpectedly, there was substantial discordance between brain MRI and MRA findings in the SWiTCH cohort. Almost all children had abnormalities in the parenchyma, with evidence of both cortical and subcortical disease. However, some children had abnormal MRI findings with vasculopathy scores of 0, whereas others had widespread vessel disease with scores of 5 or 6. Moser et al described small vessel borderzone subcortical white matter ischemia/infarction that could arise from vaso-occlusion or temporarily decreased perfusion. Cortical infarcts likely come from large branch occlusions, whereas subcortical infarcts come from smaller branches unapparent by conventional MRA.31 Surprisingly, there was no clear pattern of vessel disease associated with MRI abnormalities (cortical and/or or subcortical infarcts). Additionally, there were no clear and consistent clinical or hematological predictors of either MRI or MRA abnormalities. As severe vasculopathy and narrowed vessels would lead to sluggish/impaired blood flow, expected but previously unreported correlations between MRA findings and low or undetectable TCD velocities were observed.
Although optimized blood transfusions decrease the risk of second stroke, 2 recent publications suggest that transfusions do not prevent progression of underlying cerebral vasculopathy by MRA.40,41 Our current data support this observation: vascular and parenchymal damage was substantial and widespread on MRI, and the high rate of low or uninterpretable TCD examinations supports the likelihood that vascular disease worsened over time, despite chronic transfusion therapy. Further, despite years of chronic transfusions, this cohort had a high incidence of TIA and recurrent stroke before SWiTCH enrollment. Potential reasons for the failure of transfusions to prevent cerebrovascular disease progression are speculative, but include incomplete suppression of HbS, occasional nonadherence with the regimen, lack of impact on the elevated white blood cell count, and paradoxical decreases in oxygen delivery due to compensatory changes in vascular resistance.42 A genetic predisposition toward thrombosis or inflammation is also possible.
The SWiTCH trial demonstrated that, at least in the short term, hydroxyurea therapy is less effective in preventing stroke recurrence than transfusions. The long-term impact on vasculopathy, however, might be possible; hydroxyurea combined with transfusion therapy might even be beneficial.43 The preponderance of severe vasculopathy and brain injury in our cohort just 7 years after an initial stroke may bode poorly for prolonged stroke-free survival.
In summary, the SWiTCH cohort represents a large multicenter neuroimaging dataset with standardized high-quality prospective collection of brain MRI, MRA, and TCD. The findings document severe abnormalities in children with SCA and previous stroke, despite chronic transfusion therapy for more than 7 years on average. Our results provide insights into the pathophysiology of cerebrovascular disease in this clinical setting by revealing diffuse and extensive stenoses in many cases. Our novel standardized vasculopathy grading scale warrants further investigation to validate its utility and possible use in clinical trials to document temporal changes and therapeutic effects. The ultimate goal of preventing cerebrovascular disease among children with SCA will require prospective imaging protocols, along with early and aggressive therapeutic intervention.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the staff of the Medical Coordinating Center and the Statistics and Data Management Center for support throughout the study. The authors also appreciate the efforts of the study consultants, central laboratory personnel, site investigators, and study coordinators, plus nursing staff at each participating institution. The authors provide special recognition for the numerous sacrifices made by the children and families who participated in this study.
The clinical trial was supported by National Heart, Lung and Blood Institute grants U01-HL078787 (to R.E.W.) and U01-HL078987 (to R.W.H.).
Authorship
Contribution: K.J.H., R.J.A., W.H.S., and R.E.W. designed the study, analyzed the results, and wrote the manuscript; K.L.K. and A.L. provided data analysis; and B.A., C.D., M.M.H., S.M.J., L.K., S.T.M., and S.A.S. analyzed the results and helped write the manuscript on behalf of the entire team of SWiTCH investigators. A list of the SWiTCH investigators can be found in the supplemental Appendix.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kathleen J. Helton, Department of Radiological Sciences, St. Jude Children’s Research Hospital, 262 Danny Thomas Blvd, MS 220, Memphis, TN 38105-3678; e-mail: Kathleen.Helton@stjude.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal