Key Points
Measurement of platelet diameters in 376 patients resulted in a new classification of inherited thrombocytopenias based on platelet size.
Measurement of platelet diameters is a useful tool for differential diagnosis of inherited thrombocytopenias.
Abstract
Abnormalities of platelet size are one of the distinguishing features of inherited thrombocytopenias (ITs), and evaluation of blood films is recommended as an essential step for differential diagnosis of these disorders. Nevertheless, what we presently know about this subject is derived mainly from anecdotal evidence. To improve knowledge in this field, we evaluated platelet size on blood films obtained from 376 patients with all 19 forms of IT identified so far and found that these conditions differ not only in mean platelet diameter, but also in platelet diameter distribution width and the percentage of platelets with increased or reduced diameters. On the basis of these findings, we propose a new classification of ITs according to platelet size. It distinguishes forms with giant platelets, with large platelets, with normal or slightly increased platelet size, and with normal or slightly decreased platelet size. We also measured platelet diameters in 87 patients with immune thrombocytopenia and identified cutoff values for mean platelet diameter and the percentage of platelets with increased or reduced size that have good diagnostic accuracy in differentiating ITs with giant platelets and with normal or slightly decreased platelet size from immune thrombocytopenia and all other forms of IT.
Introduction
Until recently, inherited thrombocytopenias (ITs) were considered exceedingly rare, and only a few forms were known. The advancement of knowledge about these disorders in recent years identified several new forms, which were found to be much more frequent than the diseases known up to the end of the last century. Better knowledge of ITs changed our view of their clinical characteristics and showed that thrombocytopenia is mild in most cases and is often identified for the first time in adult life.1 If no other family members are known to be thrombocytopenic, the patients are at risk of being misdiagnosed with immune thrombocytopenic purpura (ITP). In fact, a number of patients with ITs receiving undue treatments for a wrong diagnosis of ITP have been reported.1
Early diagnosis of ITs is important not only to avoid unnecessary treatments but also to define both prognosis and therapy for patients. In fact, some genetic defects that cause congenital thrombocytopenia expose patients to the risk of developing diseases that are fatal if not treated appropriately. For instance, mutations of RUNX1 and ANKRD26, which are responsible for familial platelet disorder (FPD) with predisposition to acute myeloid leukemia (AML) and ANKRD26-related thrombocytopenia (ANKRD26-RT), respectively, greatly increase the risk of myeloid malignancies, whereas mutations of MPL, which is responsible for congenital amegakaryocytic thrombocytopenia (CAMT), always evolves into a bone marrow aplasia before adulthood.2 Thus, recognizing these disorders allow physicians to tailor follow-up to the specific risk profiles of each patient and to be ready to perform bone marrow transplantation as soon as required. It has also been shown that the thrombopoietin mimetic eltrombopag increases platelet count in most patients with MYH9-related disease (MYH9-RD).3 Thus, making the correct diagnosis allows physicians to use eltrombopag instead of platelet transfusions to prepare patients for surgery or invasive procedures.4 Making a definite diagnosis can therefore improve the management of patients with ITs.
Platelet size is unanimously recognized as one of the most important parameters for determining the genetic origin of thrombocytopenia and guiding the differential diagnosis of the various specific conditions, in that abnormalities of platelet dimensions have been described in the majority of ITs. Nevertheless, using platelet size for differentiation is poorly defined for two main reasons. First, the rarity of ITs makes it difficult to directly compare platelet size in patients with different disorders. Second, both impedentiometric and optical cell counters systematically underestimate platelet volume (as well as platelet count) in inherited macrothrombocytopenias because of their inability to recognize very large platelets.5 Moreover, instruments that operate on different principles induce variability into the measurement of platelet volume in patients with nonmacrocytic thrombocytopenias as well as in healthy participants, thus making it difficult to compare values obtained in different centers.6 As a consequence, what we presently know about this subject is derived mainly from case reports, series of patients with one form of IT, and two small studies that directly compared platelet size in some of the known ITs.5,7
To advance knowledge in this field (taking into account the present limitations of cell counters), we performed a collaborative study to measure platelet size by image analysis of peripheral blood smears obtained from patients affected by all 19 forms of IT currently known.
Patients and methods
Patients
In all, 376 patients with 19 forms of IT, 87 patients with ITP, and 55 healthy participants have been studied. IT patients were from 10 different countries (Argentina, Germany, Greece, Holland, Italy, Japan, United Kingdom, United States, Spain, and Switzerland), although the vast majority (80%) were from Italy. All ITP patients and control participants were Italian.
The main characteristics of the investigated participants are reported in Table 1. Diagnosis of ITs was always confirmed by the identification of causative mutations. Since genotype/phenotype studies in MYH9-RD revealed that clinical and laboratory abnormalities are more severe in patients with mutations in the head domain of the molecule than in those with mutations in the tail domain,8,9 these 2 categories of patients were analyzed separately. Concerning Bernard-Soulier syndrome (BSS), we classified as biallelic BSS (bBSS) those patients with homozygous or double heterozygous mutations of GPIbα, GPIbβ, or GPIX, who presented with the classical form characterized by thrombocytopenia and severe platelet dysfunction. Patients with monoallelic mutations presenting with thrombocytopenia were classified as monoallelic BSS (mBSS).2 Of note, 103 of 117 patients with mBSS had the p.Ala156Val substitution in GPIbα (Bolzano mutation), which is frequent in the Italian population.10
Number of investigated participants and their main characteristics
| Disorder . | Abbreviation in main text . | Phenotype MIM number . | No. of investigated participants . | Age (y) . | F . | M . | Platelet count × 109/L . | ||
|---|---|---|---|---|---|---|---|---|---|
| Mean . | SD . | Mean . | SD . | ||||||
| Bernard-Soulier syndrome | BSS | 231200 | 127 | 31 | 18.8 | 69 | 58 | 82 | 33.7 |
| Biallelic BSS | bBSS | 13 | 26 | 16.8 | 8 | 5 | 41 | 34.6 | |
| Monoallelic BSS | mBSS | 114 | 32 | 19 | 61 | 53 | 87 | 30.2 | |
| MYH9-related disease | MYH9-RD | 600208 | 125 | 33 | 19.3 | 59 | 66 | 35 | 25.9 |
| MYH9-RD tail mutation | tMYH9-RD | 100 | 35 | 19.9 | 48 | 52 | 39 | 26 | |
| MYH9-RD head mutation | hMYH9-RD | 25 | 26 | 15.2 | 11 | 14 | 19 | 18.6 | |
| ANKRD26-related thrombocytopenia | ANKRD26-RT | 188000 | 58 | 40 | 20.7 | 27 | 31 | 43 | 28.4 |
| ACTN1-related thrombocytopenia | ACTN1-RT | 615193 | 20 | 36 | 21.5 | 14 | 6 | 87 | 31.7 |
| Wiskott-Aldrich syndrome/ X-linked thrombocytopenia | WAS/XLT | 301000/ 313900 | 9 | 20 | 16.7 | 0 | 9 | 61 | 64.7 |
| Congenital amegakaryocytic thrombocytopenia | CAMT | 604498 | 5 | 4 | 2.7 | 2 | 3 | 13 | 4.7 |
| Gray platelet syndrome | GPS | 139090 | 5 | 37 | 31.5 | 0 | 5 | 55 | 21.3 |
| ITGA2B/ITGB3-related thrombocytopenia | ITGA2B/B3-RT | 187800 | 5 | 28 | 15.9 | 5 | 0 | 106 | 44.9 |
| TUBB1-related thrombocytopenia | TUBB1-RT | 613112 | 5 | 20 | 12.6 | 2 | 3 | 82 | 44.7 |
| Familial platelet disorder and predisposition to acute myeloid leukemia | FPD-AML | 601399 | 4 | 20 | 13.5 | 2 | 2 | 103 | 35.9 |
| CYCS-related thrombocytopenia | CYCS-RT | 612004 | 3 | 32 | 14 | 1 | 2 | 104 | 61.7 |
| FLNA-related thrombocytopenia | FLNA-RT | 2 | 16 | 20.5 | 2 | 0 | 34 | 12.7 | |
| Thrombocytopenia Paris-Trousseau | TCPT | 188025 | 2 | 0.2 | 0.06 | 1 | 1 | 49 | 9.9 |
| GFI1B-related thrombocytopenia | GFI1B-RT | 187900 | 2 | 15 | 0 | 1 | 1 | 97 | 24.7 |
| Congenital thrombocytopenia with radioulnar synostosis | CTRUS | 605432 | 1 | 10 | 0 | 1 | 30 | ||
| von Willebrand disease platelet-type | VWDP | 177820 | 1 | 30 | 1 | 0 | 130 | ||
| Thrombocytopenia with absent radii | TAR | 274000 | 1 | 6 | 1 | 0 | 19 | ||
| X-linked thrombocytopenia with thalassemia | XLTT | 314050 | 1 | 8 | 0 | 1 | 75 | ||
| ITP | 87 | 38 | 26.4 | 50 | 37 | 48 | 31.1 | ||
| Controls | 55 | 37 | 17 | 29 | 26 | 257 | 52.3 | ||
| Disorder . | Abbreviation in main text . | Phenotype MIM number . | No. of investigated participants . | Age (y) . | F . | M . | Platelet count × 109/L . | ||
|---|---|---|---|---|---|---|---|---|---|
| Mean . | SD . | Mean . | SD . | ||||||
| Bernard-Soulier syndrome | BSS | 231200 | 127 | 31 | 18.8 | 69 | 58 | 82 | 33.7 |
| Biallelic BSS | bBSS | 13 | 26 | 16.8 | 8 | 5 | 41 | 34.6 | |
| Monoallelic BSS | mBSS | 114 | 32 | 19 | 61 | 53 | 87 | 30.2 | |
| MYH9-related disease | MYH9-RD | 600208 | 125 | 33 | 19.3 | 59 | 66 | 35 | 25.9 |
| MYH9-RD tail mutation | tMYH9-RD | 100 | 35 | 19.9 | 48 | 52 | 39 | 26 | |
| MYH9-RD head mutation | hMYH9-RD | 25 | 26 | 15.2 | 11 | 14 | 19 | 18.6 | |
| ANKRD26-related thrombocytopenia | ANKRD26-RT | 188000 | 58 | 40 | 20.7 | 27 | 31 | 43 | 28.4 |
| ACTN1-related thrombocytopenia | ACTN1-RT | 615193 | 20 | 36 | 21.5 | 14 | 6 | 87 | 31.7 |
| Wiskott-Aldrich syndrome/ X-linked thrombocytopenia | WAS/XLT | 301000/ 313900 | 9 | 20 | 16.7 | 0 | 9 | 61 | 64.7 |
| Congenital amegakaryocytic thrombocytopenia | CAMT | 604498 | 5 | 4 | 2.7 | 2 | 3 | 13 | 4.7 |
| Gray platelet syndrome | GPS | 139090 | 5 | 37 | 31.5 | 0 | 5 | 55 | 21.3 |
| ITGA2B/ITGB3-related thrombocytopenia | ITGA2B/B3-RT | 187800 | 5 | 28 | 15.9 | 5 | 0 | 106 | 44.9 |
| TUBB1-related thrombocytopenia | TUBB1-RT | 613112 | 5 | 20 | 12.6 | 2 | 3 | 82 | 44.7 |
| Familial platelet disorder and predisposition to acute myeloid leukemia | FPD-AML | 601399 | 4 | 20 | 13.5 | 2 | 2 | 103 | 35.9 |
| CYCS-related thrombocytopenia | CYCS-RT | 612004 | 3 | 32 | 14 | 1 | 2 | 104 | 61.7 |
| FLNA-related thrombocytopenia | FLNA-RT | 2 | 16 | 20.5 | 2 | 0 | 34 | 12.7 | |
| Thrombocytopenia Paris-Trousseau | TCPT | 188025 | 2 | 0.2 | 0.06 | 1 | 1 | 49 | 9.9 |
| GFI1B-related thrombocytopenia | GFI1B-RT | 187900 | 2 | 15 | 0 | 1 | 1 | 97 | 24.7 |
| Congenital thrombocytopenia with radioulnar synostosis | CTRUS | 605432 | 1 | 10 | 0 | 1 | 30 | ||
| von Willebrand disease platelet-type | VWDP | 177820 | 1 | 30 | 1 | 0 | 130 | ||
| Thrombocytopenia with absent radii | TAR | 274000 | 1 | 6 | 1 | 0 | 19 | ||
| X-linked thrombocytopenia with thalassemia | XLTT | 314050 | 1 | 8 | 0 | 1 | 75 | ||
| ITP | 87 | 38 | 26.4 | 50 | 37 | 48 | 31.1 | ||
| Controls | 55 | 37 | 17 | 29 | 26 | 257 | 52.3 | ||
F, female; M, male; MIM, Mammalian Inheritance in Man.
The diagnosis of ITP was made according to guidelines of the American Society of Hematology and the British Society for Haematology,11,12 and it was confirmed by evaluating subsequent clinical evolution and response to therapy.
The institutional review board of the Istituto Di Ricovero e Cura a Carattere Scientifico Policlinico San Matteo Foundation of Pavia, Italy, approved the study protocol. The study was performed in accordance with the Declaration of Helsinki, and each center complied with local ethical rules.
Methods
Platelet count.
Platelet count was measured by the cell counters available in each center. It is worth mentioning that cell counters are at risk of underestimating platelet count in macrothrombocytopenias5 and that, therefore, it is likely that reported data overestimated the degree of thrombocytopenia in the ITs with large platelets.
Platelet diameters.
Blood smears were prepared in each center from nonanticoagulated blood obtained by finger stick and were shipped to the center that measured platelet diameters (University of Pavia- Istituto Di Ricovero e Cura a Carattere Scientifico Policlinico San Matteo Foundation, Pavia, Italy). Platelet diameters were measured by optical microscopy on May-Grünwald-Giemsa–stained blood films and by software-assisted image analysis (Axiovision 4.5; Carl Zeiss, Göttingen, Germany). On average, 200 platelets were evaluated in each patient, and the maximum diameter of each platelet was recorded.7 Platelets in clumps or those with extending pseudopodia were not considered. Technicians who measured the diameters were blinded to the patients’ diagnoses.
To evaluate the possibility of measuring the percentage of large platelets (see “Statistical analysis”) without the support of software-assisted image analysis, this parameter was calculated empirically by blinded operators who visually compared the diameters of platelets with those of red cells. All blood films examined by software-assisted image analysis were also evaluated by this empirical approach.
Statistical analysis
Analyzed parameters were mean platelet diameters (MPDs), platelet diameter distribution width (PDDW) as the 97.5th to 2.5th percentiles difference, platelet diameter large cell ratio (PDLCR) as the mean percentage of platelets above the 97.5th percentile of platelet diameter distribution in controls, and platelet diameter small cell ratio (PDSCR) as the mean percentage of platelets below the 2.5th percentile of platelet diameter distribution in controls. Descriptive statistics were computed as means, standard deviations, and 2.5th to 97.5th percentiles, decomposing the standard deviation into between and within components to take into account the hierarchical structure of data (platelet diameter measurements within patients). Correlation analysis was used to assess resemblance between measuring PDLCR with or without the support of software-assisted image analysis and to determine whether age has any impact on platelet size parameters. Receiver operating characteristic analysis was performed to identify optimal cutoffs of the analyzed parameters for discriminating specific forms of ITs from all other examined pathological conditions. Stata MP 11.1 along with nrocarea package for receiver operating characteristic analysis was used for computation.
Results
Table 2 reports the mean values of MPD, PDDW, PDLCR, and PDSCR measured in investigated conditions together with their 95% CIs.
Characteristics of platelet diameters in investigated participants
| Characteristic . | MPD (μm) . | PDDW (μm) . | PDLCR (%) . | PDSCR (%) . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean . | 95% CI . | 2.5th-97.5th . | 2.5th 95% CI . | 97.5th 95% CI . | Mean . | 95% CI . | Mean . | 95% CI . | Mean . | 95% CI . | |
| MYH9-RD | 4.51 | 4.2 to 4.8 | 2.3-8.1 | 2.2 to 2.3 | 7.8 to 8.5 | 5.9 | 5.6 to 6.2 | 58.3 | 54.8 to 61.8 | 0.5 | 0.2 to 0.8 |
| hMYH9-RD | 5.64 | 4.8 to 6.4 | 2.7-10.4 | 2.4 to 2.9 | 9.5 to 11.4 | 7.8 | 6.9 to 8.7 | 75.3 | 66.2 to 84.4 | 0.1 | 0 to 0.2 |
| tMYH9-RD | 4.28 | 4 to 4.6 | 2.1-7.6 | 2.1 to 2.2 | 7.3 to 7.8 | 5.4 | 5.1 to 5.7 | 54 | 50.7 to 57.3 | 0.6 | 0.2 to 1 |
| TUBB1-RT | 3.91 | 2.8 to 5.1 | 2.1-7.2 | 1.9 to 2.2 | 5.9 to 8.6 | 5.2 | 3.5 to 6.8 | 43.1 | 20.3 to 65.9 | 0 | |
| GPS | 3.76 | 2.7 to 4.8 | 2-7.2 | 1.8 to 2.2 | 6.6 to 7.9 | 5.2 | 4.2 to 6.2 | 36.5 | 22.5 to 50.6 | 0.4 | −0.6 to 1.4 |
| FLNA-RT | 3.75 | 2.2 to 5.3 | 2.1-6.4 | 1.6 to 2.6 | 5.5 to 7.4 | 4.3 | 1.2 to 7.4 | 38.3 | 0.3 to 76.2 | 0.4 | −4.9 to 5.8 |
| GFI1b-RT | 3.59 | 2.2 to 5 | 1.9-5.8 | 1.9 to 1.9 | 5.3 to 6.4 | 3.9 | 0.2 to 7.7 | 34.8 | −17.5 to 87.1 | 1.2 | −1.9 to 4.3 |
| BSS | 3.44 | 3.3 to 3.6 | 2-5.6 | 1.9 to 2 | 5.4 to 5.8 | 3.6 | 3.5 to 3.8 | 29.6 | 26.2 to 33 | 1.5 | 0.9 to 2.2 |
| bBSS | 4.24 | 3.7 to 4.8 | 2.5-6.6 | 2.3 to 2.7 | 6.2 to 7 | 4.1 | 3.7 to 4.4 | 58.1 | 48 to 68.2 | 0.1 | −0.1 to 0.2 |
| mBSS | 3.39 | 3.2 to 3.6 | 1.9-5.5 | 1.8 to 2 | 5.4 to 5.7 | 3.6 | 3.5 to 3.8 | 27.3 | 24 to 30.6 | 1.8 | 1 to 2.5 |
| ITGA2B/ITGB3-RT | 3.39 | 2.5 to 4.2 | 2-5.7 | 1.6 to 2.4 | 4.7 to 6.7 | 3.7 | 2.6 to 4.7 | 23.9 | 5.8 to 41.9 | 1.2 | −1 to 3.5 |
| ACTN1-RT | 3.36 | 2.9 to 3.8 | 1.8-5.8 | 1.7 to 1.9 | 5.5 to 6.2 | 4.1 | 3.8 to 4.4 | 25.5 | 19.4 to 31.5 | 1.6 | 0.6 to 2.6 |
| FPD-AML | 3.18 | 2.2 to 4.2 | 1.6-5.6 | 1.4 to 1.8 | 4.9 to 6.2 | 3.9 | 3.1 to 4.8 | 18.6 | 5.3 to 31.9 | 3.1 | −0.9 to 7.1 |
| TCPT | 3.15 | 1.9 to 4.4 | 1.7-5.5 | 1.4 to 2.1 | 4 to 7.1 | 3.8 | −4.4 to 12.0 | 24.6 | −225.1 to 274.2 | 1.8 | −8.6 to 12.3 |
| XLTT | 3.02 | 0.9 to 5.1 | 1.3-5.8 | 1.2 to 1.6 | 5.2 to 6.3 | 4.5 | 16.3 | 5.1 | |||
| ANKRD26-RT | 2.93 | 2.7 to 3.1 | 1.8-4.8 | 1.7 to 1.9 | 4.6 to 5.1 | 3.1 | 2.8 to 3.3 | 12.8 | 9.3 to 16.2 | 1.6 | 1 to 2.1 |
| CTRUS | 2.79 | 1.6 to 3.9 | 1.8-4.3 | 1.7 to 1.9 | 3.8 to 5.3 | 2.5 | 3.5 | 0 | |||
| VWDP | 2.74 | 1.6 to 3.9 | 1.7-4.4 | 1.4 to 1.9 | 3.7 to 5.1 | 2.7 | 3.3 | 1.1 | |||
| TAR | 2.35 | 1.3 to 3.4 | 1.4-3.4 | 1.4 to 1.6 | 3.1 to 4.4 | 2 | 0.8 | 6.3 | |||
| CAMT | 2.32 | 1.8 to 2.9 | 1.3-4 | 0.9 to 1.8 | 3.1 to 5 | 2.7 | 1.8 to 3.5 | 6.4 | −9.4 to 22.2 | 18.9 | −5.2 to 42.9 |
| CYCS-RT | 2.28 | 1.5 to 3.1 | 1.2-4.4 | 0.8 to 1.6 | 3.3 to 5.4 | 3.1 | 0.9 to 5.7 | 4.3 | −4 to 12.6 | 18.8 | −19.2 to 56.7 |
| WAS/XLT | 2.25 | 1.9 to 2.6 | 1.4-3.8 | 1.2 to 1.5 | 3.5 to 4.1 | 2.4 | 2.2 to 2.6 | 2.3 | −0.1 to 4.8 | 10.3 | 3.2 to 17.3 |
| ITP | 3.11 | 2.9 to 3.3 | 1.8-5.2 | 1.7 to 1.8 | 5 to 5.4 | 3.5 | 3.3 to 3.7 | 16.4 | 13.6 to 19.2 | 2.8 | 1.3 to 4.2 |
| Controls | 2.58 | 2.4 to 2.7 | 1.6-3.9 | 1.5 to 1.6 | 3.7 to 4.1 | 2.3 | 2.2 to 2.5 | 3.5 | 2.2 to 4.8 | 4.7 | 3.1 to 6.3 |
| Characteristic . | MPD (μm) . | PDDW (μm) . | PDLCR (%) . | PDSCR (%) . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean . | 95% CI . | 2.5th-97.5th . | 2.5th 95% CI . | 97.5th 95% CI . | Mean . | 95% CI . | Mean . | 95% CI . | Mean . | 95% CI . | |
| MYH9-RD | 4.51 | 4.2 to 4.8 | 2.3-8.1 | 2.2 to 2.3 | 7.8 to 8.5 | 5.9 | 5.6 to 6.2 | 58.3 | 54.8 to 61.8 | 0.5 | 0.2 to 0.8 |
| hMYH9-RD | 5.64 | 4.8 to 6.4 | 2.7-10.4 | 2.4 to 2.9 | 9.5 to 11.4 | 7.8 | 6.9 to 8.7 | 75.3 | 66.2 to 84.4 | 0.1 | 0 to 0.2 |
| tMYH9-RD | 4.28 | 4 to 4.6 | 2.1-7.6 | 2.1 to 2.2 | 7.3 to 7.8 | 5.4 | 5.1 to 5.7 | 54 | 50.7 to 57.3 | 0.6 | 0.2 to 1 |
| TUBB1-RT | 3.91 | 2.8 to 5.1 | 2.1-7.2 | 1.9 to 2.2 | 5.9 to 8.6 | 5.2 | 3.5 to 6.8 | 43.1 | 20.3 to 65.9 | 0 | |
| GPS | 3.76 | 2.7 to 4.8 | 2-7.2 | 1.8 to 2.2 | 6.6 to 7.9 | 5.2 | 4.2 to 6.2 | 36.5 | 22.5 to 50.6 | 0.4 | −0.6 to 1.4 |
| FLNA-RT | 3.75 | 2.2 to 5.3 | 2.1-6.4 | 1.6 to 2.6 | 5.5 to 7.4 | 4.3 | 1.2 to 7.4 | 38.3 | 0.3 to 76.2 | 0.4 | −4.9 to 5.8 |
| GFI1b-RT | 3.59 | 2.2 to 5 | 1.9-5.8 | 1.9 to 1.9 | 5.3 to 6.4 | 3.9 | 0.2 to 7.7 | 34.8 | −17.5 to 87.1 | 1.2 | −1.9 to 4.3 |
| BSS | 3.44 | 3.3 to 3.6 | 2-5.6 | 1.9 to 2 | 5.4 to 5.8 | 3.6 | 3.5 to 3.8 | 29.6 | 26.2 to 33 | 1.5 | 0.9 to 2.2 |
| bBSS | 4.24 | 3.7 to 4.8 | 2.5-6.6 | 2.3 to 2.7 | 6.2 to 7 | 4.1 | 3.7 to 4.4 | 58.1 | 48 to 68.2 | 0.1 | −0.1 to 0.2 |
| mBSS | 3.39 | 3.2 to 3.6 | 1.9-5.5 | 1.8 to 2 | 5.4 to 5.7 | 3.6 | 3.5 to 3.8 | 27.3 | 24 to 30.6 | 1.8 | 1 to 2.5 |
| ITGA2B/ITGB3-RT | 3.39 | 2.5 to 4.2 | 2-5.7 | 1.6 to 2.4 | 4.7 to 6.7 | 3.7 | 2.6 to 4.7 | 23.9 | 5.8 to 41.9 | 1.2 | −1 to 3.5 |
| ACTN1-RT | 3.36 | 2.9 to 3.8 | 1.8-5.8 | 1.7 to 1.9 | 5.5 to 6.2 | 4.1 | 3.8 to 4.4 | 25.5 | 19.4 to 31.5 | 1.6 | 0.6 to 2.6 |
| FPD-AML | 3.18 | 2.2 to 4.2 | 1.6-5.6 | 1.4 to 1.8 | 4.9 to 6.2 | 3.9 | 3.1 to 4.8 | 18.6 | 5.3 to 31.9 | 3.1 | −0.9 to 7.1 |
| TCPT | 3.15 | 1.9 to 4.4 | 1.7-5.5 | 1.4 to 2.1 | 4 to 7.1 | 3.8 | −4.4 to 12.0 | 24.6 | −225.1 to 274.2 | 1.8 | −8.6 to 12.3 |
| XLTT | 3.02 | 0.9 to 5.1 | 1.3-5.8 | 1.2 to 1.6 | 5.2 to 6.3 | 4.5 | 16.3 | 5.1 | |||
| ANKRD26-RT | 2.93 | 2.7 to 3.1 | 1.8-4.8 | 1.7 to 1.9 | 4.6 to 5.1 | 3.1 | 2.8 to 3.3 | 12.8 | 9.3 to 16.2 | 1.6 | 1 to 2.1 |
| CTRUS | 2.79 | 1.6 to 3.9 | 1.8-4.3 | 1.7 to 1.9 | 3.8 to 5.3 | 2.5 | 3.5 | 0 | |||
| VWDP | 2.74 | 1.6 to 3.9 | 1.7-4.4 | 1.4 to 1.9 | 3.7 to 5.1 | 2.7 | 3.3 | 1.1 | |||
| TAR | 2.35 | 1.3 to 3.4 | 1.4-3.4 | 1.4 to 1.6 | 3.1 to 4.4 | 2 | 0.8 | 6.3 | |||
| CAMT | 2.32 | 1.8 to 2.9 | 1.3-4 | 0.9 to 1.8 | 3.1 to 5 | 2.7 | 1.8 to 3.5 | 6.4 | −9.4 to 22.2 | 18.9 | −5.2 to 42.9 |
| CYCS-RT | 2.28 | 1.5 to 3.1 | 1.2-4.4 | 0.8 to 1.6 | 3.3 to 5.4 | 3.1 | 0.9 to 5.7 | 4.3 | −4 to 12.6 | 18.8 | −19.2 to 56.7 |
| WAS/XLT | 2.25 | 1.9 to 2.6 | 1.4-3.8 | 1.2 to 1.5 | 3.5 to 4.1 | 2.4 | 2.2 to 2.6 | 2.3 | −0.1 to 4.8 | 10.3 | 3.2 to 17.3 |
| ITP | 3.11 | 2.9 to 3.3 | 1.8-5.2 | 1.7 to 1.8 | 5 to 5.4 | 3.5 | 3.3 to 3.7 | 16.4 | 13.6 to 19.2 | 2.8 | 1.3 to 4.2 |
| Controls | 2.58 | 2.4 to 2.7 | 1.6-3.9 | 1.5 to 1.6 | 3.7 to 4.1 | 2.3 | 2.2 to 2.5 | 3.5 | 2.2 to 4.8 | 4.7 | 3.1 to 6.3 |
ITs are listed in descending order of MPD.
CI, confidence interval.
MPD
Figure 1A shows MPDs measured by software-assisted image analysis in different groups of patients and controls, together with the 25th to 75th percentiles and the most extreme values. The categories of patients are reported from left to right in descending order of the medians of MPDs. Patients with MYH9-RD due to mutations in the head domain of MYH9 had the highest MPD with a value of 5.64 μm. Interestingly, MPD was much lower in patients with MYH9-RD deriving from mutations in the tail domain of MYH9 who had MPD similar to that measured in bBSS. MYH9-RD and bBSS were the only ITs with MPDs larger than 4 μm. Results for ITP patients are located to the left of those for healthy participants, and only Wiskott-Aldrich syndrome (WAS)/X-linked thrombocytopenia (XLT), CAMT, and CYCS-related thrombocytopenia (CYCS-RT) are to the right results for controls. WAS and XLT derive from mutations in the WAS gene, but they are often considered separate entities because of differences in their clinical phenotypes. Moreover, it has been reported that splenectomy increased platelet size as well as platelet count of affected patients.13 On this basis, we analyzed patients with WAS and XLT separately, separating splenectomized patients from non-splenectomized ones. Although MPDs were slightly larger in XLT than in WAS as well in splenectomized patients versus non-splenectomized patients, all these differences were subtle and far from being statistically significant. Thus, for the purposes of this study, we considered all patients with WAS mutations as belonging to a single category.
Platelet size parameters. (A) MPDs, (B) PDDWs and (C) PDLCRs in ITs, immune thrombocytopenia, and controls. Disorders with only 1 examined patient are not reported. Lines in the middle of the box are the medians, boxes are defined by the 25th and 75th percentiles (quartile 1 [Q1] and quartile 3 [Q3]), the ends of the whiskers are the most extreme values within Q3 + 1.5(Q3 − Q1) and Q1− 1.5(Q3 − Q1), respectively, and dots are outliers. The dashed horizontal lines indicate the best cutoff values for distinguishing ITs with giant platelets (upper lines) and those with normal or slightly decreased platelet size (lower lines) from the other forms of ITs and immune thrombocytopenia.
Platelet size parameters. (A) MPDs, (B) PDDWs and (C) PDLCRs in ITs, immune thrombocytopenia, and controls. Disorders with only 1 examined patient are not reported. Lines in the middle of the box are the medians, boxes are defined by the 25th and 75th percentiles (quartile 1 [Q1] and quartile 3 [Q3]), the ends of the whiskers are the most extreme values within Q3 + 1.5(Q3 − Q1) and Q1− 1.5(Q3 − Q1), respectively, and dots are outliers. The dashed horizontal lines indicate the best cutoff values for distinguishing ITs with giant platelets (upper lines) and those with normal or slightly decreased platelet size (lower lines) from the other forms of ITs and immune thrombocytopenia.
Because a mild increase (about 5%) in platelet volume has been observed in patients older than age 60 years vs those younger than age 18 years,14 we searched for correlation between age and MPD both in the entire population of investigated patients and in the disorders with more than 8 patients. No significant correlation has been found (data not shown), suggesting that age differences in different categories of investigated patients did not significantly interfere with the results of platelet diameter measurement.
PDDW
PDDWs in different conditions are reported in Figure 1B. Comparison of MPDs in Figure 1A-B indicates that PDDWs and MPDs are largely related values, in that PDDWs increase with increasing MPDs both in ITs and ITP. The lowest value of PDDW has been observed in controls.
PDLCR
As shown in Figure 1C, the analysis of the percentage of large platelets in different conditions provided interesting results. PDLCR, defined as the proportion of platelets with diameters larger than the 97.5th percentile of MPD in healthy participants (>3.9 μm) was above 5% in the vast majority of ITs as well as in ITP. In particular, 11 and 3 ITs had median PDLCRs larger than 20% and 50%, respectively. The increase in large platelets was clearly related to the increase in platelet diameters, but differences with respect to controls were larger for PDLCRs than MPDs, although wide variations of PDLCRs were observed within patients with the same disorder. Of note, low values of PDLCRs were observed in ITs with the smallest MPDs.
Empirical evaluation by a blinded operator of the percentages of platelets larger than 3.9 μm (half the diameter of red cells) in patients and controls showed excellent correlation with the values obtained by software-assisted image analysis (Pearson’s r = 0.95; P < .0001).
PDSCR
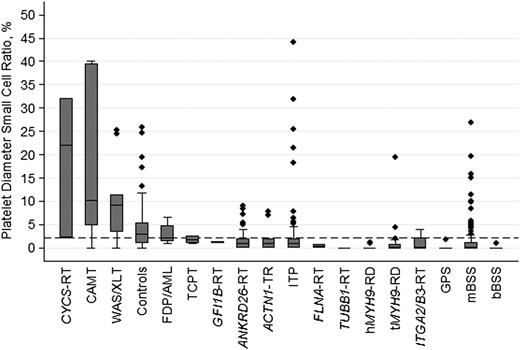
Concerning PDSCR, defined as the percentage of platelets smaller than the 2.5th percentile of MPD in healthy participants (<1.6 μm), the highest values have been observed in CYCS-RT, CAMT, and WAS/XLT, with values of 18.8%, 18.9%, and 10.3%, respectively (Figure 2).
PDSCRs in ITs, immune thrombocytopenia, and controls. Disorders with only 1 examined patient are not reported. Line in the middle of the box is the median, box is defined by the 25th and 75th percentiles (Q1 and Q3), and the ends of the whiskers are the most extreme values within Q3 + 1.5(Q3 − Q1) and Q1 − 1.5(Q3 − Q1), respectively. The dashed horizontal line indicates the best cutoff value for distinguishing ITs with normal or slightly decreased platelet size from the other forms of ITs and immune thrombocytopenia.
PDSCRs in ITs, immune thrombocytopenia, and controls. Disorders with only 1 examined patient are not reported. Line in the middle of the box is the median, box is defined by the 25th and 75th percentiles (Q1 and Q3), and the ends of the whiskers are the most extreme values within Q3 + 1.5(Q3 − Q1) and Q1 − 1.5(Q3 − Q1), respectively. The dashed horizontal line indicates the best cutoff value for distinguishing ITs with normal or slightly decreased platelet size from the other forms of ITs and immune thrombocytopenia.
Platelet diameters for differential diagnosis between ITs and ITP
Given the results of the descriptive analysis, we wondered whether platelet diameters could play a role in differentiating ITs from ITP. Evaluation of the data reported in Table 2 and in Figures 1-2 indicates that MPD, PDDW, PDLCR, and PDSCR in many ITs were largely overlapping with those observed in ITP. Notable exceptions were represented by a head mutation in MYH9-RD (hMYH9-RD), a tail mutation in MYH9-RD (tMYH9-RD), and bBSS, which had values of MPDs, PDDWs, and PDLCRs above and rather well separated from those measured in ITP. Conversely, the values of MPDs, PDDWs, and PDLCR in WAS/XLT, CAMT, and CYCS-RT were below those measured in ITP. These ITs were also characterized by PDLCRs higher than those in ITP. Thus, we searched for the best cutoff values for distinguishing these conditions and measured their diagnostic accuracy. Table 3 reports the results of these analyses of ITs with large platelets.
Platelet diameters for distinguishing IT with giant platelets
| . | Cutoff . | AUC . | Sensitivity % . | 95% CI . | Specificity % . | 95% CI . |
|---|---|---|---|---|---|---|
| MPD μm | >3.74 | 0.9154 | 86 | 79 to 91 | 87 | 82 to 90 |
| PDDW μm | >4.27 | 0.8812 | 80 | 72 to 86 | 83 | 78 to 88 |
| PDLCR % | >39.80 | 0.9208 | 85 | 78 to 91 | 87 | 83 to 90 |
| MPD μm and PDLCR % | >3.74 and >39.8 | 0.9991 | 98 | 93 to 99 | 99 | 97 to 100 |
| . | Cutoff . | AUC . | Sensitivity % . | 95% CI . | Specificity % . | 95% CI . |
|---|---|---|---|---|---|---|
| MPD μm | >3.74 | 0.9154 | 86 | 79 to 91 | 87 | 82 to 90 |
| PDDW μm | >4.27 | 0.8812 | 80 | 72 to 86 | 83 | 78 to 88 |
| PDLCR % | >39.80 | 0.9208 | 85 | 78 to 91 | 87 | 83 to 90 |
| MPD μm and PDLCR % | >3.74 and >39.8 | 0.9991 | 98 | 93 to 99 | 99 | 97 to 100 |
Area under the curve (AUC), sensitivity and specificity of best cutoff levels for MPD, PDDW, and PDLCR identified by receiver operating characteristic analysis in discriminating hMYH9-RD, tMYH9-RD, and bBSS from all other examined forms of thrombocytopenia.
An MPD larger than 3.74 μm and a PDLCR higher than 39.8% had good sensitivity and specificity in differentiating hMYH9-RD, tMYH9-RD, and bBSS from the other forms of ITs and ITP. Moreover, combining these two cutoff levels increased sensitivity to 98% and specificity to 99%.
Table 4 provides results concerning differentiation of WAS/XLT, CAMT, CYCS-RT, and thrombocytopenia with absent radii (TAR) from all other ITs and ITP. An MPD below 2.62 μm, a PDLCR below 9.62%, and a PDSCR above 2.11% had good discriminating capacity in terms of sensitivity and specificity. Combining MPD and PDLCR, sensitivity rose to 96% and specificity rose to 93%.
Cutoff values of platelet diameters for distinguishing IT with normal or slightly decreased platelet size
| . | Cutoff . | AUC . | Sensitivity % . | 95% CI . | Specificity % . | 95% CI . |
|---|---|---|---|---|---|---|
| MPD μm | <2.62 | 0.9217 | 83 | 58 to 96 | 90 | 87 to 93 |
| PDDW μm | <2.85 | 0.8333 | 78 | 52 to 93 | 83 | 79 to 86 |
| PDLCR % | <9.62 | 0.9202 | 94 | 71 to 100 | 80 | 76 to 84 |
| PDSCR % | >2.11 | 0.8763 | 83 | 58 to 96 | 86 | 82 to 89 |
| MPD μm and PDLCR % | <2.62 and <7.21 | 0.9852 | 96 | 87 to 99 | 93 | 90 to 95 |
| . | Cutoff . | AUC . | Sensitivity % . | 95% CI . | Specificity % . | 95% CI . |
|---|---|---|---|---|---|---|
| MPD μm | <2.62 | 0.9217 | 83 | 58 to 96 | 90 | 87 to 93 |
| PDDW μm | <2.85 | 0.8333 | 78 | 52 to 93 | 83 | 79 to 86 |
| PDLCR % | <9.62 | 0.9202 | 94 | 71 to 100 | 80 | 76 to 84 |
| PDSCR % | >2.11 | 0.8763 | 83 | 58 to 96 | 86 | 82 to 89 |
| MPD μm and PDLCR % | <2.62 and <7.21 | 0.9852 | 96 | 87 to 99 | 93 | 90 to 95 |
AUC, sensitivity and specificity of best cutoff levels for MPD, PDDW, PDLCR, and PDSCR identified by receiver operating characteristic analysis in discriminating WAS/XLT, CYCS-RT, CAMT, and TAR from all other forms of thrombocytopenia.
Discussion
Centralized evaluation of peripheral blood films in a large number of patients with different ITs made a direct comparison of platelet diameters in all disorders identified so far possible for the first time.
Analysis of MPDs confirmed that most ITs have large platelets, although the magnitude of the difference with respect to healthy participants was moderate in most cases, and only patients with hMYH9-RD had an MPD more than twice that of the controls. MPD was largely above the upper limit of normal range (as defined by the 97.5th percentiles of platelet diameters in healthy participants: 3.9 μm) only in MYH9-RD (in patients with mutations in both the head and the tail of the molecule) and bBSS. Of note, hMYH9-RD, tMYH9-RD, and bBSS had the highest percentage of large platelets (platelet diameters above the upper limit of normal range), with PDLCRs ranging from 54% to 75%. For classification purposes, we therefore consider it appropriate to describe these conditions as characterized by very large MPDs and a very high proportion of large platelets. More briefly, we can describe these ITs as characterized by giant platelets.
Concerning the other ITs, in 7 forms MPDs were higher than the upper limit of standard deviation of this parameter in healthy participants (3.2 μm): TUBB1-related thrombocytopenia (TUBB1-RT), gray platelet syndrome (GPS), FLNA-related thrombocytopenia (FLNA-RT), GFI1b-related thrombocytopenia (GFIb-RT), mBSS, ITGA2B/B3-related thrombocytopenia (ITGA2B/B3-RT), and ACTN1-related thrombocytopenia (ACTN1-RT). In all these conditions, PDLCRs were higher than 20%. Thus, we propose to define these ITs as characterized by large MPDs and a high proportion of large platelets. Otherwise, we can define them as characterized by large platelets.
In FPD-AML, thrombocytopenia Paris-Trousseau (TCPT), XLT with thalassemia (XLTT), ANKRD26-RT, congenital thrombocytopenia with radioulnar synostosis (CTRUS), and von Willebrand disease platelet type (VWDP), MPD was higher than in healthy participants but did not differ by more than 25% from the average values of controls. PDLCRs were higher or similar to those of controls. Taking into account the high variability of values within each category of patients, we suggest that these disorders can be classified as characterized by normal or slightly increased platelet size, with normal or increased proportion of large platelets. In brief, these ITs have normal or slightly increased platelet size.
TAR, CAMT, CYCS-RT, and WAS/XLT had MPDs below that of controls, and the percentage of PDSCRs was higher than in healthy participants. However, the ranges of values were largely overlapping with those of controls. On the basis of these findings, we believe that these conditions may be described as having normal or slightly reduced platelet size with a normal or slightly increased percentage of small platelets. For classification purposes, TAR, CAMT, CYCS-RT, and WAS/XLT may be defined as having normal or slightly decreased platelet size.
Table 5 reports the new classification that emerged from the precise measurement of platelet diameters and the percentage of platelets larger or smaller than specific threshold values. We emphasize that it identifies distinct categories of diseases, whereas in reality, the size of the platelets in ITs varies in a continuous manner from values much higher to values slightly lower than those of controls. Moreover, platelet size varies in patients with the same disorder. Thus, this classification, as with many other disease classifications, is rather artificial and is intended to be used as a scholastic tool. We also note that, for some disorders, we were able to examine only a few patients, or even only one. Thus, a definitive classification of these very rare forms requires the evaluation of additional patients.
Classification of ITs according to MPDs and the percentage of large platelets
| ITs . | MPD (μm) . | Large platelets (%) . | . |
|---|---|---|---|
| With giant platelets | >4 | PDLCR >50% | hMYH9-RD |
| bBSS | |||
| tMYH9-RD | |||
| With large platelets | >3.2 | PDLCR >20% | TUBB1-RT |
| GPS | |||
| FLNA-RT | |||
| GFI1b-RT | |||
| mBSS | |||
| ITGA2B/B3-RT | |||
| ACTN1-RT | |||
| With normal or slightly increased platelet size | >2.6 | And/or PDLCR >5% | FDP-AML |
| TCPT | |||
| XLTT | |||
| ANKRD26-RT | |||
| CTRUS | |||
| VWDP | |||
| With normal or slightly decreased platelet size | <2.6 | And/or SDCR >5% | TAR |
| CAMT | |||
| CYCS-RT | |||
| XLT/WAS |
| ITs . | MPD (μm) . | Large platelets (%) . | . |
|---|---|---|---|
| With giant platelets | >4 | PDLCR >50% | hMYH9-RD |
| bBSS | |||
| tMYH9-RD | |||
| With large platelets | >3.2 | PDLCR >20% | TUBB1-RT |
| GPS | |||
| FLNA-RT | |||
| GFI1b-RT | |||
| mBSS | |||
| ITGA2B/B3-RT | |||
| ACTN1-RT | |||
| With normal or slightly increased platelet size | >2.6 | And/or PDLCR >5% | FDP-AML |
| TCPT | |||
| XLTT | |||
| ANKRD26-RT | |||
| CTRUS | |||
| VWDP | |||
| With normal or slightly decreased platelet size | <2.6 | And/or SDCR >5% | TAR |
| CAMT | |||
| CYCS-RT | |||
| XLT/WAS |
Our study aimed to better define platelet phenotypes in ITs and also to evaluate whether platelet diameters may be used as a diagnostic tool for differential diagnosis of ITs. This matter has been addressed by two studies that measured MPDs in 35 and 46 patients with inherited macrothrombocytopenias and evaluated the ability of platelet diameters to discriminate between these conditions and ITP. The first study found that an MPD larger than 3.3 μm differentiated MYH9-RT, mBSS, and bBSS from ITP with 89% sensitivity and 88% specificity.7 The second study, evaluating a different patient population, found that the same cutoff value had 67% specificity and 75% sensitivity in differentiating these conditions from ITP.5
This study confirmed that MPD has good diagnostic accuracy in distinguishing ITs with giant platelets (hMYH9-RD, tMYH9-RD, and bBSS) not only from ITP but also from all other ITs. Indeed, an MPD larger than 3.74 μm had 86% sensitivity and 87% specificity in this respect. Of note mBSS, in most cases deriving from a Bolzano mutation, was not included within the group of disorders distinguishable from ITP by this parameter because MPD of affected patients was lower in this large case series than in previous smaller studies.5,7 The results were quite similar to MPD measured in ITP. A new finding of our study was the demonstration that MPD was able to distinguish ITs with normal or slightly decreased platelet size from ITP and the other forms of ITs. In fact, an MPD smaller than 2.62 μm had 83% sensitivity and 90% specificity in this regard.
Another new finding was that the percentage of large platelets had good diagnostic accuracy in differentiating ITs with giant platelets from all other investigated forms of thrombocytopenia. In particular, a percentage of PDLCRs higher than 39.8% had 85% sensitivity and 87% specificity in this respect. Moreover, combining an MPD larger than 3.7 μm and a PDLCR higher than 39.8% increased sensitivity and specificity to near 100%.
We also showed that both PDSCR and PDLCR can be used to recognize ITs with normal or slightly decreased platelet size. A PDSCR above 2.11% had 83% sensitivity and 86% specificity in differentiating these forms from ITP and all other ITs, whereas a PDLCR below 9.62% had 94% sensitivity and 80% specificity. The joint use of MPD and PDLCR increased sensitivity and specificity to 96% and 93%, respectively.
Thus, in our series of 376 patients with ITs, evaluation of blood films would have been a valuable diagnostic tool for recognizing the 156 patients with giant platelets or normal to slightly decreased platelet size.
An obvious objection to the proposal of using platelet diameters for diagnostic purposes is that measuring this parameter by microscope evaluation of blood films is time consuming and, as a consequence, difficult to apply in clinical practice. The availability of cell counters that reliably measure platelet size and the percentage of platelets larger or smaller than specific cutoff values would probably solve this problem. As already discussed, these instruments are not yet available, and we have to wait for the next generation of instruments to verify whether they are as efficient as microscope evaluation of blood films in differential diagnosis of ITs. In the meantime, we have to continue using the microscope for assessing platelet dimensions. This task is made easier by the fortunate coincidence that 3.9 μm, the cutoff value for large platelets, is about half the diameter of normal erythrocytes on blood films. Of note, results of empirical evaluation of PDLCR by visually comparing the diameters of platelets with those of red cells showed a very strong correlation with the values obtained by software-assisted image analysis. Comparing the size of platelets with those of erythrocytes may therefore be a simple and quick method for getting a first impression of PDLCR and to decide whether a more accurate evaluation of platelet diameter might be worthwhile. Indeed, a PDLCR higher than 40% or smaller than 10% supports a diagnosis of an IT with giant platelets and an IT with normal or slightly decreased platelet size, respectively, whereas PDLCRs within these two limits are compatible with ITP as well as with ITs with large platelets and with normal or slightly increased platelet size.
One flaw in our study is that we studied only one or a very few patients with some forms of ITs. Because of the high variability of platelet diameters observed in each IT, it may be that the values we reported here are not fully representative of these poorly investigated disorders. Thus, it might happen that the proposed classification of ITs according to platelet diameters is amended in the future by studying additional patients. Statistical analyses also suffered from the dearth of some forms of IT, and the study of additional patients is therefore required to better define the size of platelets in rare disorders. Finally, we cannot exclude that differences in the preparation of blood films in different centers affected the evaluation of platelet diameters.
In conclusion, the precise measurement of platelet diameters in the largest series of patients with ITs ever collected and including all known disorders allowed us to propose a new classification of these disorders based on platelet size. Moreover, it allowed us to identify MPD, as well as the percentage of large and small platelets, as useful parameters for differential diagnosis of ITs.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grant no. GGP10089 from Telethon Foundation Italy.
Authorship
Contribution: P.N., A.P., A.S., M.S., G.L., G.R., L.D.N., P.G., P.G.H., N.P.-M., S.K., M.C., J.B., E.D.C., U.R., and F.F. investigated patients and provided study materials; E.C., F.M., V.B., C.C., and S.B. analyzed blood films; E.C. assembled data; G.B. analyzed data; P.N., G.B., A.P., and C.L.B. wrote the manuscript; and all authors had access to primary clinical data and revised and gave final approval to the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Carlo L. Balduini, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico Policlinico San Matteo, Viale Golgi 19, Pavia, Italy; e-mail: c.balduini@smatteo.pv.it.

![Figure 1. Platelet size parameters. (A) MPDs, (B) PDDWs and (C) PDLCRs in ITs, immune thrombocytopenia, and controls. Disorders with only 1 examined patient are not reported. Lines in the middle of the box are the medians, boxes are defined by the 25th and 75th percentiles (quartile 1 [Q1] and quartile 3 [Q3]), the ends of the whiskers are the most extreme values within Q3 + 1.5(Q3 − Q1) and Q1− 1.5(Q3 − Q1), respectively, and dots are outliers. The dashed horizontal lines indicate the best cutoff values for distinguishing ITs with giant platelets (upper lines) and those with normal or slightly decreased platelet size (lower lines) from the other forms of ITs and immune thrombocytopenia.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/6/10.1182_blood-2014-03-564328/4/m_e4f1.jpeg?Expires=1767710628&Signature=mPGSloppKqeKcPWao13Nd-qG5yNrjRQR2MLE~DLJ9nj19NE4lgGoR6Hcxm72oeAG6xkqLnW0Tzj~9hXUUZEJUNfbtjEUumkPNWD1e6krz-Ns7kEOAmVjgvWVZioI8cW0bn9ItJr7O8JeE5k2sfIKDnnOX2sowmimZzcYosFEDEMDEPCsN22kqDXufEBJJjItdKtG-pOHqGT5sKNysFCeoNwSpTd3kzoms1XUvzh7KLsYM7o~TaSUaSEGE2Baa55pfYyD2-gyd-m8sJm-4wyLddrjDM~uLjOBad3v9Nvk-qOk73v3dA5mZlyRmI1R6x7iHVYlKIhjSjrp3pk3K0z9IA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal