Abstract
The new oral anticoagulants (NOACs), which include dabigatran, rivaroxaban, apixaban, and edoxaban, are poised to replace warfarin for treatment of the majority of patients with venous thromboembolism (VTE). With a rapid onset of action and the capacity to be administered in fixed doses without routine coagulation monitoring, NOACs streamline VTE treatment. In phase 3 trials in patients with acute symptomatic VTE, NOACs have been shown to be noninferior to conventional anticoagulant therapy for prevention of recurrence and are associated with less bleeding. Rivaroxaban and dabigatran are already licensed for VTE treatment in the United States, and apixaban and edoxaban are under regulatory consideration for this indication. As the number of approved drugs increases, clinicians will need to choose the right anticoagulant for the right VTE patient. To help with this decision, this review (1) compares the pharmacologic profiles of the NOACs, (2) outlines the unique design features of the phase 3 trials that evaluated the NOACs for VTE treatment, (3) reviews the results of these trials highlighting similarities and differences in the findings, (4) provides perspective about which VTE patients should receive conventional treatment or are candidates for NOACs, and (5) offers suggestions about how to choose among the NOACs.
Introduction
Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), occurs for the first time in about 1 in 1000 persons each year, and the incidence rises with age to at least 5 in 1000 persons in those over the age of 80 years.1,2 About one-third of patients with symptomatic VTE present with PE, whereas the remainder manifests with DVT.3 Within 1 month of diagnosis, death occurs in approximately 6% of patients with DVT and 12% of those with PE.4 Although VTE often occurs after surgery, with immobilization, or in patients with cancer, as much as 50% of VTE patients have no identifiable risk factors and are identified as having unprovoked VTE.5 If anticoagulant therapy is stopped in patients with unprovoked VTE, the risk of recurrence is at least 10% at 1 year and 30% at 5 years.6 Recurrent DVT increases the risk of postthrombotic syndrome, a chronic disorder that occurs in 20% to 50% of DVT patients and is characterized by leg swelling and discomfort; venous ulcers can develop in severe cases.7 Chronic thromboembolic pulmonary hypertension develops in 2% to 4% of patients with PE and can be life threatening.8 Therefore, VTE is a common disorder associated with significant morbidity and mortality.
Anticoagulants are the cornerstone of VTE treatment. The goal of therapy is to prevent thrombus extension or embolization and to prevent new thrombi from forming. Traditionally, treatment occurs in 2 overlapping steps.9 It starts with a rapidly-acting parenteral anticoagulant, usually low-molecular-weight heparin (LMWH), which is overlapped with a vitamin K antagonist, such as warfarin. As initial therapy, the parenteral anticoagulant is given for at least 5 days and is stopped when the anticoagulant response with warfarin is therapeutic, as evidenced by an international normalized ratio (INR) between 2 and 3. Warfarin is then continued as long-term therapy for a minimum of 3 months. At this point, the decision to stop or continue treatment depends on the balance between the risk of recurrence if warfarin is stopped and the risk of bleeding if it is continued. Patients with VTE in the setting of transient and reversible risk factors, such as surgery, have a low risk of recurrence if anticoagulant therapy is stopped at 3 months provided they are fully mobile.5 In contrast, those with ongoing risk factors, such as active cancer, and patients with unprovoked VTE are often prescribed extended anticoagulation therapy as long as the bleeding risk is not excessive. Therefore, anticoagulant treatment of VTE has been divided into 3 stages: initial therapy, long-term treatment, and extended anticoagulation.
Although current therapy is effective and safe, it is a cumbersome process for patients and physicians because LMWH needs to be administered via daily subcutaneous injections, and warfarin requires frequent coagulation monitoring and dose adjustments to ensure that the INR remains therapeutic. The limitations of warfarin prompted the development of new oral anticoagulants (NOACs), which can be given in fixed doses and produce such a predictable anticoagulant response that routine monitoring is unnecessary. Because of their rapid onset of action, the NOACs have the potential to enable all-oral regimens, which can replace parenteral anticoagulants and warfarin for initial, long-term, and extended VTE treatment.
Four NOACs have been evaluated for VTE treatment: dabigatran (an oral thrombin inhibitor) and rivaroxaban, apixaban, and edoxaban, which are oral factor Xa inhibitors. All 4 agents have been compared with conventional anticoagulant therapy for the treatment of acute symptomatic VTE, and all but edoxaban have been compared with placebo for extended treatment. Dabigatran has also been compared with warfarin for extended therapy. Rivaroxaban is currently licensed for initial, long-term, and extended VTE treatment, whereas dabigatran is approved for the latter 2 indications. With apixaban and edoxaban currently under consideration for licensing for the VTE indication, it is likely that we will soon have 2 additional choices. Focusing on VTE, this paper (1) compares and contrasts the pharmacologic properties of the NOACs with those of warfarin; (2) highlights how differences in the design of the phase 3 clinical trials impact how each of the NOACs will be used; (3) describes the results of these trials, pointing out the class effects with the NOACs, and the findings that potentially differentiate one agent from another; (4) provides perspectives on the emerging role of the NOACs for treatment of VTE with a focus on identification, of which patients are or are not candidates for the new agents; and (5) offers suggestions on how to choose among the NOACs.
Comparison of the pharmacologic properties of NOACs with those of warfarin
As is outlined in Table 1, warfarin acts as an anticoagulant by reducing the function of the vitamin K–dependent clotting proteins—factors II, VII, IX, and X—thereby attenuating the extrinsic, intrinsic, and common pathways of blood coagulation. Because of its indirect mechanism of action, the onset and offset of action with warfarin take several days. In contrast, the NOACs inhibit only a single target—either factor Xa or thrombin—and have a rapid onset of action such that peak plasma levels are achieved 1 to 4 hours after oral administration.10 With half-lives of about 12 hours, the NOACs also have a rapid offset of action.
Comparison of the pharmacologic properties of warfarin, rivaroxaban, apixaban, and edoxaban
| . | Warfarin . | Dabigatran . | Rivaroxaban . | Apixaban . | Edoxaban . |
|---|---|---|---|---|---|
| Target | VKORC1 | Thrombin | Factor Xa | Factor Xa | Factor Xa |
| Prodrug | No | Yes | No | No | No |
| Bioavailability (%) | 100 | 7 | 80 | 60 | 62 |
| Dosing | OD | BID | OD (BID) | BID | OD |
| Time-to-peak effect | 4-5 d | 1-3 h | 2-4 h | 1-2 h | 1-2 h |
| Half-life (h) | 40 | 14-17 | 7-11 | 8-14 | 5-11 |
| Renal clearance as unchanged drug (%) | None | 80 | 33 | 27 | 50 |
| Monitoring | Yes | No | No | No | No |
| Interactions | Multiple | P-gp | 3A4/P-gp | 3A4/P-gp | P-gp |
| . | Warfarin . | Dabigatran . | Rivaroxaban . | Apixaban . | Edoxaban . |
|---|---|---|---|---|---|
| Target | VKORC1 | Thrombin | Factor Xa | Factor Xa | Factor Xa |
| Prodrug | No | Yes | No | No | No |
| Bioavailability (%) | 100 | 7 | 80 | 60 | 62 |
| Dosing | OD | BID | OD (BID) | BID | OD |
| Time-to-peak effect | 4-5 d | 1-3 h | 2-4 h | 1-2 h | 1-2 h |
| Half-life (h) | 40 | 14-17 | 7-11 | 8-14 | 5-11 |
| Renal clearance as unchanged drug (%) | None | 80 | 33 | 27 | 50 |
| Monitoring | Yes | No | No | No | No |
| Interactions | Multiple | P-gp | 3A4/P-gp | 3A4/P-gp | P-gp |
OD, once daily; BID, twice daily; P-gp, P-glycoprotein; VKORC1, C1 subunit of the vitamin K epoxide reductase enzyme; 3A4, cytochrome P450 3A4 isoenzyme.
Although warfarin is predominantly cleared through nonrenal mechanisms, the NOACs are excreted, at least in part via the kidneys. The extent of renal clearance varies depending on the agent: about 80% of dabigatran is cleared unchanged by the kidneys, and 50%, 33%, and 27% of edoxaban, rivaroxaban, and apixaban, respectively, are cleared unchanged via the renal route. Because of their renal clearance, NOACs should be used with caution in patients with a creatinine clearance <30 mL/min and should not be used in patients with a creatinine clearance <15 mL/min.
The dose of warfarin varies among patients, reflecting differences in dietary vitamin K intake, multiple drug-drug interactions, and common polymorphisms that affect warfarin metabolism or pharmacodynamics. Furthermore, warfarin has a narrow therapeutic window and in patients with VTE, deficient anticoagulation can lead to recurrent thrombosis, whereas excessive anticoagulation can cause bleeding. Consequently, frequent coagulation monitoring and dose adjustments are necessary to ensure that the INR remains within the therapeutic range. In contrast, because the NOACs produce a more predictable anticoagulant response, they can be given in fixed doses without routine monitoring, thereby simplifying VTE treatment. There are few clinically important drug-drug interactions with the NOACs, although potent inhibitors or inducers of CYP 3A4 and/or p-glycoprotein can be problematic, and there are no dietary restrictions, except that rivaroxaban should be administered with a meal to maximize its absorption.
Vitamin K is the antidote for warfarin. When given orally or by slow intravenous infusion, vitamin K will restore the INR to baseline levels, but this often takes more than 24 hours. Rapid warfarin reversal can be achieved with 4-factor prothrombin complex concentrate (PCC). Fresh frozen plasma is an alternative to PCC, but plasma infusion takes longer and large volumes are often needed, which can be problematic for patients with compromised cardiopulmonary function. There are no specific antidotes for the NOACs, but these are under development.11,12 Although PCC may be effective for reversal of the NOACs, clinical data are limited.13
Comparison of the designs of the clinical trials evaluating NOACs for VTE treatment
The studies comparing the NOACs with conventional therapy for initial and long-term VTE treatment and those comparing NOACs with placebo or warfarin for extended therapy vary in design. The trial designs for the 2 indications will be discussed separately.
Initial and long-term VTE treatment
Dabigatran, rivaroxaban, apixaban, and edoxaban were compared with conventional treatment in the RE-COVER I and II, EINSTEIN-DVT and PE, AMPLIFY, and Hokusai-VTE trials, respectively.14-19 The designs of these trials are summarized in Table 2. Except for EINSTEIN, which used a prospective, randomized, open-label, blinded end point evaluation (PROBE) design, all of the trials were conducted in a double-blind fashion. A list of expanded clinical trial abbreviations can be found in the Appendix.
Design and patient characteristics of the trials comparing NOACs with conventional therapy for acute VTE treatment
| . | Dabigatran . | Rivaroxaban . | Apixaban . | Edoxaban . | ||
|---|---|---|---|---|---|---|
| Trial | RE-COVER I & II | EINSTEIN | AMPLIFY | Hokusai-VTE | ||
| Indication | VTE | DVT | PE | VTE | VTE | |
| Design | Double-blind | PROBE | Double-blind | Double-blind | ||
| Number of patients | 2539 | 2568 | 3449 | 4832 | 5365 | 8240 |
| Mean age ± SD (y) | 54.9 ± 16.0 | 56.1 ± 16.4 | 57.7 ± 7.3 | 57.0 ± 16.0 | 55.8 ± 16.3 | |
| CrCl <30 mL/min, n (%) | 22 (0.4) | 15 (0.4) | 6 (0.1) | 29 (0.5) | n/a | |
| Age ≥75 y, n (%) | 529 (10) | 440 (13) | 843 (17) | 768 (14) | 1104 (13) | |
| Prior VTE (%) | 22 | 19 | 20 | 16 | 18 | |
| Unprovoked VTE (%) | 35 | 62.0 | 64.5 | 89.8 | 65.7 | |
| Index event PE ± DVT (%) | 31 | 0.7 | 100 | 34 | 40 | |
| Noninferiority margin | 2.75 | 2.0 | 1.8 | 1.5 | ||
| Bridge with heparin/LMWH | Yes | No | No | Yes | ||
| Treatment protocol | 150 mg BID | 15 mg BID for 3 wk; then 20 mg OD | 10 mg BID for 7 d; then 5 mg BID | 60 mg OD; 30 mg OD for those with a creatinine clearance of 30-50 mL/min, weight <60 kg, or taking potent P-gp inhibitors | ||
| Duration (mo) | 6 | 3, 6, 12 | 6 | 3-12 | ||
| TTR (%) | 60 | 58 | 63 | 61 | 64 | |
| . | Dabigatran . | Rivaroxaban . | Apixaban . | Edoxaban . | ||
|---|---|---|---|---|---|---|
| Trial | RE-COVER I & II | EINSTEIN | AMPLIFY | Hokusai-VTE | ||
| Indication | VTE | DVT | PE | VTE | VTE | |
| Design | Double-blind | PROBE | Double-blind | Double-blind | ||
| Number of patients | 2539 | 2568 | 3449 | 4832 | 5365 | 8240 |
| Mean age ± SD (y) | 54.9 ± 16.0 | 56.1 ± 16.4 | 57.7 ± 7.3 | 57.0 ± 16.0 | 55.8 ± 16.3 | |
| CrCl <30 mL/min, n (%) | 22 (0.4) | 15 (0.4) | 6 (0.1) | 29 (0.5) | n/a | |
| Age ≥75 y, n (%) | 529 (10) | 440 (13) | 843 (17) | 768 (14) | 1104 (13) | |
| Prior VTE (%) | 22 | 19 | 20 | 16 | 18 | |
| Unprovoked VTE (%) | 35 | 62.0 | 64.5 | 89.8 | 65.7 | |
| Index event PE ± DVT (%) | 31 | 0.7 | 100 | 34 | 40 | |
| Noninferiority margin | 2.75 | 2.0 | 1.8 | 1.5 | ||
| Bridge with heparin/LMWH | Yes | No | No | Yes | ||
| Treatment protocol | 150 mg BID | 15 mg BID for 3 wk; then 20 mg OD | 10 mg BID for 7 d; then 5 mg BID | 60 mg OD; 30 mg OD for those with a creatinine clearance of 30-50 mL/min, weight <60 kg, or taking potent P-gp inhibitors | ||
| Duration (mo) | 6 | 3, 6, 12 | 6 | 3-12 | ||
| TTR (%) | 60 | 58 | 63 | 61 | 64 | |
n/a, not available; OD, once daily; BID, twice daily; P-gp, P-glycoprotein; LMWH, low-molecular-weight heparin; PROBE, prospective, randomized, open-label, blinded endpoint; TTR, time in therapeutic range with warfarin.
The primary efficacy outcome of these trials was recurrent VTE. Although all of the studies were designed to show noninferiority of the NOACs compared with conventional therapy, the noninferiority margins differed and ranged from 1.5 to 2.75. The primary safety outcome in these trials was major bleeding, or the composite of major or clinically relevant nonmajor bleeding.
In patients with acute VTE, the risk of recurrence is highest in the first month after diagnosis.20 Phase 2 VTE treatment studies with rivaroxaban and apixaban not only helped to identify the dose to be carried into phase 3 but also provided reassurance that an all-oral approach was possible from the start.21,22 Consequently, the EINSTEIN and AMPLIFY trials used oral regimens that started with more intensive therapy; with rivaroxaban, this involved administering 15 mg twice daily for 3 weeks followed by 20 mg daily thereafter, whereas the apixaban regimen consisted of 10 mg twice daily for 7 days followed by 5 mg twice daily thereafter. Phase 2 studies in VTE treatment were not conducted with dabigatran or edoxaban; doses for the RE-COVER and Hokusai-VTE trials were selected based on the results of phase 2 studies in patients with atrial fibrillation.23,24 In the absence of evidence supporting the immediate use of dabigatran or edoxaban in patients with acute VTE, the treatment regimens in the RE-COVER and Hokusai-VTE trials started with a minimum of a 5-day course of a parenteral anticoagulant, and patients were then transitioned to warfarin or given dabigatran or edoxaban, respectively.
Treatment duration varied among the trials; it was fixed at 6 months in the RE-COVER and AMPLIFY trials, and it was flexible at 3, 6, or 12 months in the EINSTEIN and Hokusai-VTE trials. In the EINSTEIN trials, investigators selected the treatment duration when patients were enrolled; in Hokusai-VTE, investigators determined treatment duration in an ongoing fashion. Treatment duration can be an important design variable; a flexible treatment period more closely mirrors current practice where treatment duration depends on patient characteristics. In contrast, fixing treatment duration at 6 months may limit enrollment of patients with provoked VTE because guidelines recommend a 3-month course of anticoagulant treatment of such patients provided that their reversible risk factors have resolved.9 This explains why the majority of patients enrolled in the AMPLIFY trial had unprovoked VTE, but it does not explain the under-representation of such patients in the RE-COVER I and II trials (Table 2).
The case fatality rate in patients with PE is twice that in those with DVT.2 Consequently, it was important to recruit sufficient numbers of PE patients into these trials to ensure that the efficacy and safety of the NOACS in PE and DVT patients were similar. In RE-COVER, AMPLIFY, and Hokusai-VTE, this was accomplished by ensuring that at least 30% of the enrolled patients had PE with or without concomitant DVT. In contrast, in the EINSTEIN program, patients with PE, with or without associated DVT, and those with DVT alone were enrolled into separate trials. All of the trials excluded PE patients with hemodynamic compromise because such patients often undergo advanced therapies, which may include administration of fibrinolytic agents.
Although the clinical characteristics of patients enrolled in the various trials differed, few patients with a creatinine clearance <30 mL/min were included, and the proportion of patients over the age of 75 years ranged from only 10% to 17% (Table 2). Heavier patients were better represented than underweight patients in all of the studies.
Extended VTE treatment
Rivaroxaban, apixaban, and dabigatran were compared with placebo in the double-blind EINSTEIN-Extension, AMPLIFY-Extension, and RE-SONATE trials, respectively (Table 3).16,25,26 Patients enrolled in the EINSTEIN and AMPLIFY-Extension studies had received 6 to 12 months of anticoagulation therapy for their index VTE event, whereas those entered in the RE-SONATE study had received 6 to 18 months of treatment. Although single-dose regimens of rivaroxaban and dabigatran were evaluated in the EINSTEIN-Extension and RE-SONATE trials (20 mg once daily and 150 mg twice daily, respectively), 2 dosage regimens of apixaban were studied in the AMPLIFY-Extension trial (2.5 and 5 mg twice daily). All of the trials were powered to demonstrate superiority of the NOACs over placebo, and the primary efficacy outcome was recurrent VTE.
Comparison of the designs of the trials evaluating NOACs for extended VTE treatment
| . | Dabigatran . | Rivaroxaban . | Apixaban . | |
|---|---|---|---|---|
| Trial | RE-SONATE | RE-MEDY | EINSTEIN-Ext | AMPLIFY-Ext |
| Comparator | Placebo | Warfarin | Placebo | Placebo |
| Design | Double-blind | Double-blind | Double-blind | Double-blind |
| Number of patients | 1343 | 2856 | 1197 | 2486 |
| Noninferiority margin | — | 2.85 | — | — |
| Duration of prior anticoagulation treatment (mo) | 6-18 | 3-12 | 6-12 | 3-12 |
| Treatment protocol | 150 mg BID | 150 mg BID | 20 mg OD | 2.5 or 5 mg BID |
| Duration (mo) | 6 | 6-36 | 6-12 | 12 |
| . | Dabigatran . | Rivaroxaban . | Apixaban . | |
|---|---|---|---|---|
| Trial | RE-SONATE | RE-MEDY | EINSTEIN-Ext | AMPLIFY-Ext |
| Comparator | Placebo | Warfarin | Placebo | Placebo |
| Design | Double-blind | Double-blind | Double-blind | Double-blind |
| Number of patients | 1343 | 2856 | 1197 | 2486 |
| Noninferiority margin | — | 2.85 | — | — |
| Duration of prior anticoagulation treatment (mo) | 6-18 | 3-12 | 6-12 | 3-12 |
| Treatment protocol | 150 mg BID | 150 mg BID | 20 mg OD | 2.5 or 5 mg BID |
| Duration (mo) | 6 | 6-36 | 6-12 | 12 |
BID, twice daily; Ext, extension; OD, once daily.
Dabigatran is the only agent to be compared with warfarin for extended VTE treatment in the RE-MEDY trial.26 Patients enrolled in this trial had received 3 to 12 months of anticoagulant treatment of their index VTE event, and the study was designed to show noninferiority of dabigatran compared with warfarin with the noninferiority margin set at 2.85. Although edoxaban has yet to be evaluated in an extension study, because of the flexible treatment duration in the Hokusai-VTE trial, the drug has been administered for up to 12 months.
Results of clinical trials
Initial VTE treatment
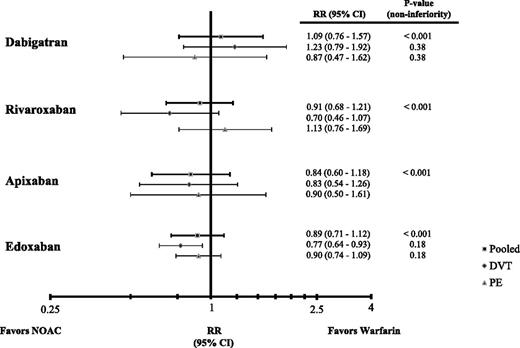
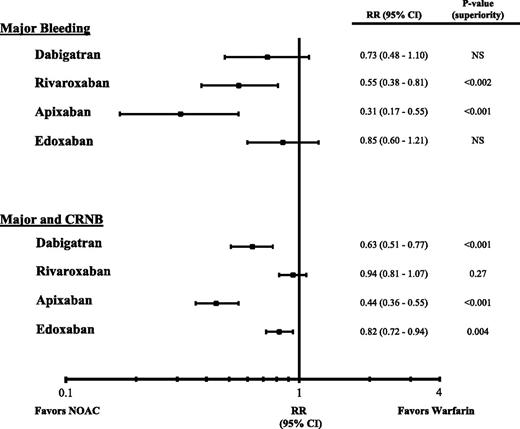
In patients with acute symptomatic VTE, rates of recurrent VTE with the NOACs are similar to those with conventional therapy (Table 415,18,19,27 ) in both PE and DVT patients (Figure 1). The consistency of the findings across trials is reassuring and suggests that as a class, NOACs are noninferior to conventional therapy for treatment of PE and/or DVT. Rates of major bleeding are significantly lower with rivaroxaban and apixaban than with conventional therapy, and with the exception of rivaroxaban, the rates of the composite of major or clinically relevant nonmajor bleeding also are significantly lower with the NOACs than with conventional therapy (Figure 2).27,28 The absolute risk reductions in safety shown in Table 4 translate to the prevention of one major or clinically relevant nonmajor bleed in every 19 to 167 patients treated with a NOAC instead of warfarin. Overall, therefore, NOACs are noninferior to conventional therapy for VTE treatment and are associated with less bleeding. Furthermore, NOACs are more convenient to administer. Rivaroxaban and apixaban can be given in all-oral regimens that obviate the need for a parenteral anticoagulant at the outset, and all of the NOACs are easier to administer than warfarin because they can be given in fixed doses without the need for routine coagulation monitoring.
Efficacy and safety outcomes of the trials comparing NOACs with conventional therapy for acute VTE treatment
| . | Efficacy outcome . | Safety outcomes . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Recurrent VTE and VTE-related death . | Major bleeding . | Major and CRNB . | |||||||
| NOAC, n/N(%) . | Warfarin, n/N (%) . | ARR, % (95% CI) . | NOAC, n/N (%) . | Warfarin, n/N (%) . | ARR % (95% CI) . | NOAC n/N (%) . | Warfarin, n/N (%) . | ARR % (95% CI) . | |
| Dabigatran15 | 60/2553 (2.4) | 55/2554 (2.2) | 0.2 (−0.6, 1.0) | 37/2553 (1.4) | 51/2554 (2.0) | −0.5 (−1.3, 0.2) | 136/2553 (5.3) | 217/2554 (8.5) | −3.2 (−4.6, −1.8) |
| Rivaroxaban27 | 86/4130 (2.1) | 95/4131 (2.3) | −0.2 (−0.8, 0.4) | 40/4130 (1.0) | 72/4116 (1.7) | −0.8 (−1.3, −0.3) | 388/4130 (9.4) | 412/4116 (10.0) | −0.6 (−1.9, 0.7) |
| Apixaban18 | 59/2609 (2.3) | 71/2635 (2.7) | −0.4 (−1.3, 0.4) | 15/2676 (0.6) | 49/2689 (1.8) | −1.3 (−1.8, −0.6) | 115/2676 (4.3) | 261/2689 (9.7) | −5.4 (−6.8, −4.1) |
| Edoxaban19 | 130/4118 (3.2) | 146/4122 (3.5) | −0.4 (−1.2, 0.4) | 56/4118 (1.4) | 66/4122 (1.6) | −0.2 (−0.8, 0.3) | 349/4118 (8.5) | 423/4112 (10.3) | −1.8 (−3.1, −0.6) |
| . | Efficacy outcome . | Safety outcomes . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Recurrent VTE and VTE-related death . | Major bleeding . | Major and CRNB . | |||||||
| NOAC, n/N(%) . | Warfarin, n/N (%) . | ARR, % (95% CI) . | NOAC, n/N (%) . | Warfarin, n/N (%) . | ARR % (95% CI) . | NOAC n/N (%) . | Warfarin, n/N (%) . | ARR % (95% CI) . | |
| Dabigatran15 | 60/2553 (2.4) | 55/2554 (2.2) | 0.2 (−0.6, 1.0) | 37/2553 (1.4) | 51/2554 (2.0) | −0.5 (−1.3, 0.2) | 136/2553 (5.3) | 217/2554 (8.5) | −3.2 (−4.6, −1.8) |
| Rivaroxaban27 | 86/4130 (2.1) | 95/4131 (2.3) | −0.2 (−0.8, 0.4) | 40/4130 (1.0) | 72/4116 (1.7) | −0.8 (−1.3, −0.3) | 388/4130 (9.4) | 412/4116 (10.0) | −0.6 (−1.9, 0.7) |
| Apixaban18 | 59/2609 (2.3) | 71/2635 (2.7) | −0.4 (−1.3, 0.4) | 15/2676 (0.6) | 49/2689 (1.8) | −1.3 (−1.8, −0.6) | 115/2676 (4.3) | 261/2689 (9.7) | −5.4 (−6.8, −4.1) |
| Edoxaban19 | 130/4118 (3.2) | 146/4122 (3.5) | −0.4 (−1.2, 0.4) | 56/4118 (1.4) | 66/4122 (1.6) | −0.2 (−0.8, 0.3) | 349/4118 (8.5) | 423/4112 (10.3) | −1.8 (−3.1, −0.6) |
ARR, absolute risk reduction; CRNB, clinically relevant nonmajor bleeding; n/N (%), number of events/number of patients in group (percentage).
Hazard ratios (HR) for recurrent VTE and VTE-related death and their 95% confidence intervals (CI) in phase 3 trials comparing NOACs with conventional therapy for acute VTE treatment.
Hazard ratios (HR) for recurrent VTE and VTE-related death and their 95% confidence intervals (CI) in phase 3 trials comparing NOACs with conventional therapy for acute VTE treatment.
Hazard ratios (HR) for major bleeding or major plus clinically relevant nonmajor bleeding (CRNB) and their 95% confidence intervals (CI) in phase 3 trials comparing NOACs with conventional therapy for acute VTE treatment.
Hazard ratios (HR) for major bleeding or major plus clinically relevant nonmajor bleeding (CRNB) and their 95% confidence intervals (CI) in phase 3 trials comparing NOACs with conventional therapy for acute VTE treatment.
In a predefined subset of PE patients with right ventricular dysfunction, as evidenced by right ventricular dilatation on computed tomography of the chest and/or an elevated level of N-terminal pro-brain natriuretic peptide, edoxaban was superior to warfarin. Right ventricular dysfunction occurs in patients with extensive PE, which explains the higher mortality rates reported in such patients.5 Although the mortality rates in PE patients with and without right ventricular dysfunction were similar in the Hokusai-VTE trial, the superiority of edoxaban in patients with right ventricular dysfunction supports the effectiveness of edoxaban for VTE treatment.19
Extended VTE treatment
Dabigatran, rivaroxaban, and apixaban are superior to placebo for the prevention of recurrent VTE and are associated with low rates of major bleeding.16,25,26 Compared with placebo, both the treatment and the prophylactic dose of apixaban (5 mg and 2.5 mg twice daily, respectively) significantly reduced the risk of recurrent VTE.25 Although both dose regimens were associated with low rates of major bleeding, there was a trend for less clinically relevant nonmajor bleeding with the lower-dose apixaban regimen. This finding raises the possibility that the intensity of treatment with NOACs can be lowered for extended VTE treatment to reduce the risk of bleeding without compromising efficacy. In contrast, attempts to lower the intensity of warfarin therapy for extended VTE treatment resulted in reduced efficacy without evidence of less bleeding.29
Dabigatran was noninferior to warfarin for extended VTE treatment in the RE-MEDY trial, and was associated with less bleeding.26 Although the number of events was small, myocardial infarction was more common with dabigatran than with warfarin, a phenomenon observed in other studies that compared dabigatran with warfarin.15,30,31 The clinical relevance of this finding is uncertain, however, because the rates of all-cause mortality and cardiovascular mortality tend to be lower with dabigatran than with warfarin.32
Choosing the right anticoagulant for the right VTE patient
Acute VTE
When faced with a patient with acute VTE, the first question to ask is whether the patient is suitable for treatment with a NOAC (Table 5). Patients who require advanced treatment, such as those with massive PE who are candidates for systemic or catheter-directed thrombolytic therapy or patients with extensive proximal DVT who may benefit from pharmaco-mechanical therapy, should not receive NOACs to start because such patients were excluded from the clinical trials and because the NOACs have not been evaluated in conjunction with thrombolytic therapy. Intravenous unfractionated heparin is probably the best choice for these patients because of its short half-life, the option to tailor the dose as needed, and the availability of protamine sulfate as an antidote should serious bleeding occur. For the same reasons, heparin may also be the best choice for patients at high initial risk for bleeding, such as those whose VTE occurs soon after major surgery or trauma.
Suggestions for choice of anticoagulant for acute VTE treatment
| Characteristic . | Drug choice . | Rationale . |
|---|---|---|
| Extensive DVT or massive PE | Heparin | Such patients often require advanced therapy and were excluded from trials with the NOACs |
| High initial risk of bleeding | Heparin | Enables dose titration; rapid offset and availability of protamine as an antidote simplify management should bleeding occur |
| Active cancer | LMWH | No trials comparing NOACs with LMWH |
| Pregnancy | LMWH | Warfarin and NOACs cross the placenta |
| Liver dysfunction with increased prothrombin time/INR at baseline | Warfarin | Such patients were excluded from the trials because NOACs undergo hepatic metabolism |
| Unable to afford NOACs | LMWH followed by warfarin | NOACs cost less than LMWH but are more expensive than warfarin |
| Limited access to anticoagulation clinic because of impaired mobility or geographical inaccessibility | NOAC | Given in fixed doses without monitoring |
| All-oral therapy | Rivaroxaban or apixaban | Only NOACs to be evaluated in all-oral regimens |
| Creatinine clearance <30 mL/min | Warfarin | Such patients were excluded from trials with NOACs |
| Creatinine clearance 30-50 mL/min | Rivaroxaban, apixaban, or edoxaban | Less affected by renal impairment than dabigatran; if edoxaban is chosen, the 30-mg OD dose should be used |
| Dyspepsia or upper gastrointestinal symptoms | Rivaroxaban, apixaban, or edoxaban | Dyspepsia in as much as 10% given dabigatran |
| Recent gastrointestinal bleed | Apixaban | More gastrointestinal bleeding with dabigatran, rivaroxaban, and edoxaban than with warfarin |
| Recent acute coronary syndrome | Rivaroxaban, apixaban or edoxaban | Small myocardial infarction signal with dabigatran |
| Poor compliance with long-term twice-daily dosing | Rivaroxaban or edoxaban | OD regimens for long-term use |
| Characteristic . | Drug choice . | Rationale . |
|---|---|---|
| Extensive DVT or massive PE | Heparin | Such patients often require advanced therapy and were excluded from trials with the NOACs |
| High initial risk of bleeding | Heparin | Enables dose titration; rapid offset and availability of protamine as an antidote simplify management should bleeding occur |
| Active cancer | LMWH | No trials comparing NOACs with LMWH |
| Pregnancy | LMWH | Warfarin and NOACs cross the placenta |
| Liver dysfunction with increased prothrombin time/INR at baseline | Warfarin | Such patients were excluded from the trials because NOACs undergo hepatic metabolism |
| Unable to afford NOACs | LMWH followed by warfarin | NOACs cost less than LMWH but are more expensive than warfarin |
| Limited access to anticoagulation clinic because of impaired mobility or geographical inaccessibility | NOAC | Given in fixed doses without monitoring |
| All-oral therapy | Rivaroxaban or apixaban | Only NOACs to be evaluated in all-oral regimens |
| Creatinine clearance <30 mL/min | Warfarin | Such patients were excluded from trials with NOACs |
| Creatinine clearance 30-50 mL/min | Rivaroxaban, apixaban, or edoxaban | Less affected by renal impairment than dabigatran; if edoxaban is chosen, the 30-mg OD dose should be used |
| Dyspepsia or upper gastrointestinal symptoms | Rivaroxaban, apixaban, or edoxaban | Dyspepsia in as much as 10% given dabigatran |
| Recent gastrointestinal bleed | Apixaban | More gastrointestinal bleeding with dabigatran, rivaroxaban, and edoxaban than with warfarin |
| Recent acute coronary syndrome | Rivaroxaban, apixaban or edoxaban | Small myocardial infarction signal with dabigatran |
| Poor compliance with long-term twice-daily dosing | Rivaroxaban or edoxaban | OD regimens for long-term use |
OD, once daily; LMWH, low-molecular-weight heparin.
Other groups of patients who should not receive NOACs include those with a creatinine clearance <15 mL/min and those with hepatic dysfunction. NOACs are contraindicated in patients with a creatinine clearance <15 mL/min because efficacy and safety data in such patients are lacking, and the drugs should be used with caution in patients with a creatinine clearance between 15 and 30 mL/min because few such patients were included in the trials. All of the NOACs require some degree of hepatic metabolism; consequently, they should be avoided in patients with liver dysfunction as evidenced by an increased prothrombin time/INR and reduced serum albumin at baseline, important indicators of an increased Child-Pugh score.33
Patients with VTE in the setting of active cancer for which they are receiving chemotherapy, biological agents, and/or radiation may do better with LMWH, which has been shown to be superior to warfarin for the prevention of recurrent VTE in this population.34 Although some patients with active cancer were included in the studies with the NOACs, the numbers are small and studies comparing NOACs with extended LMWH in patients with VTE in the setting of active cancer are lacking. Likewise, NOACs have not been evaluated in patients with VTE in the setting of antiphospholipid antibody syndrome (APS) or other high-risk thrombophilic conditions, or in patients who develop VTE as a complication of heparin-induced thrombocytopenia (HIT). Until more data are available, VTE patients with APS should receive conventional anticoagulant therapy and those with HIT should be given fondaparinux or a parenteral direct thrombin inhibitor. Patients who cannot afford the NOACs should receive conventional anticoagulant treatment because NOACs are more expensive than warfarin. Finally, if compliance is a concern, warfarin may be a better choice because of its need for monitoring.
In VTE patients eligible for NOACs, there is no evidence to recommend one agent over another because the NOACs have never been compared in head-to-head trials. Nonetheless, suggestions can be provided based on the distinct pharmacologic profiles of the NOACs and the designs of the trials in which they were investigated. Thus for patients with a creatinine clearance between 30 and 50 mL/min, an oral factor Xa inhibitor may be a better choice than dabigatran because the factor Xa inhibitors are less dependent on renal excretion. If edoxaban is chosen for such patients, the dose must be reduced from 60 mg to 30 mg once daily to maintain similar drug exposure. The NOACs should be avoided in patients with a creatinine clearance <30 mL/min.
An all-oral regimen streamlines transition of care from the clinic or the emergency department to home; rivaroxaban and apixaban, the only agents to be evaluated in all-oral regimens, are the agents of choice for this purpose. Selection between the two may depend on the ease of switching from the higher initial dose to the maintenance dose at 3 weeks and 1 week, respectively, and patient preference for a once daily or twice daily regimen thereafter. In contrast, dabigatran and edoxaban should only be prescribed after patients have received a minimum of a 5-day course of heparin or LMWH treatment. Rivaroxaban or apixaban may be good choices for patients over the age of 75 with reduced renal function (creatinine clearance of 30-50 mL/min), particularly females with low body weight, because the benefit-to-risk profile of these agents in frail patients is superior to that of conventional therapy.18,27 Dabigatran may not be the best choice in patients with a history of coronary artery disease because of its higher risk of myocardial infarction compared with warfarin.32 Dabigatran should be avoided in patients with upper gastrointestinal complaints because dyspepsia can occur in as much as 10% of patients treated with this agent, a problem that tends to subside over time and can often be resolved by taking the drug with food. Except for apixaban, the rate of gastrointestinal bleeding with the NOACs is higher than that with warfarin, particularly in the elderly.18,35 Consequently, apixaban may be the best choice for patients with a recent history of gastrointestinal bleeding. Finally, potential drug-drug interactions may inform the selection of NOACs. For example, as potent inducers of CYP3A4, phenytoin and carbamazepine may reduce plasma levels of apixaban or rivaroxaban but will not affect the levels of dabigatran or edoxaban.
The risk of bleeding with NOACs or warfarin is increased with concomitant use of antiplatelet agents, such as aspirin and nonsteroidal antiinflammatory drugs, and these agents should be avoided if possible. For patients who must use aspirin, the daily dose of aspirin should not exceed 100 mg.
NOACs for treatment of prevalent cases of VTE
Patients already on warfarin for VTE treatment should be switched to a NOAC if their INR is erratic. A switch can also be considered for patients who find INR testing and warfarin dose adjustment burdensome, such as those with limited mobility or patients with active lifestyles. For extended treatment, the risk of bleeding is likely to be lower with the NOACs than with warfarin, particularly if the dose intensity can be reduced, as was investigated with apixaban. Therefore, for patients who have already completed at least 6 months of anticoagulant treatment of their index VTE event, apixaban 2.5 mg twice daily is a good choice. It remains to be established whether reduced dose regimens are effective for extended therapy with the other NOACs and whether such regimens can be used in patients with a history of recurrent VTE.
Compared with placebo, aspirin produces approximately a 32% reduction in the risk of recurrence when used for secondary prevention in patients with unprovoked VTE who have received at least 6 months of anticoagulant therapy for their index event.36,37 By contrast, anticoagulants produce an 80% to 90% reduction in the risk of recurrence compared with placebo. Therefore, anticoagulants should be used instead of aspirin for secondary VTE prevention in most patients, particularly when one considers the convenience of the NOACs, their low risk of bleeding, and the fact that with the lower-dose apixaban regimen, the rates of bleeding approached those with placebo.
Potential limitations of NOACs for VTE treatment
The lack of specific antidotes for the NOACs can complicate their reversal in patients who require urgent surgery or in those with life-threatening bleeding, a concern that makes some clinicians hesitant to prescribe the NOACs.38 It is important to point out that despite the lack of antidotes, the outcome in patients with intracranial bleeds or major bleeds in other sites was no worse in patients taking dabigatran than in those taking warfarin, and intracranial bleeding was less frequent with dabigatran than with warfarin.39,40 Likewise, in patients requiring urgent surgery or interventions, the shorter half-lives of the NOACs relative to warfarin may be an advantage.41 Emerging postmarketing data suggest that the safety of the NOACs in the real world is similar to that observed in the trials.42 Nonetheless, antidotes are under development to simplify the reversal of NOACs in emergency situations.
In the absence of laboratory monitoring, adherence may be more difficult to assess with NOACs than with warfarin. Ongoing education and vigilance by physicians, nurses, and pharmacists can help to ensure adherence, and there is evidence that persistence with therapy may be better with NOACs than with warfarin.43
Finally, although cheaper than LMWH, the NOACs are more expensive than warfarin. However, the cost is likely to decrease when all 4 agents are licensed for this indication, and with a reduced risk of bleeding compared with conventional therapy, the NOACs are likely to provide a cost-effective option.44
Conclusions and future directions
The NOACs represent an important advance in VTE treatment. By streamlining transition of care, they facilitate out-of-hospital treatment and are easier to use and safer than warfarin. Despite the availability of all-oral regimens of rivaroxaban and apixaban, clinicians may be more reluctant to initiate use of them in PE patients than in those with DVT. Although more data are needed to evaluate the efficacy and safety of the NOACs in patients with PE, even if treatment in these patients starts with LMWH, it remains easier to transition and maintain them on a NOAC than on warfarin.
To enable all-oral therapy with dabigatran and edoxaban, starting doses need to be identified and their efficacy and safety determined. Although the NOACs are a potentially attractive option in patients with VTE in the setting of active cancer, they need to be compared with extended LMWH in such patients. Gastrointestinal disturbances associated with chemotherapy may limit intake of the NOACs in cancer patients, and potential drug-drug interactions may increase or decrease drug exposure. Furthermore, dose titration in cancer patients with thrombocytopenia is likely to be more complicated with NOACs than with LMWH. For these reasons, head-to-head trials comparing the NOACs with extended LMWH in cancer patients with VTE are needed.
Finally, the utility of the NOACs for VTE treatment in vulnerable patients, such as those who are morbidly obese, those of very low body weight, pregnant women, nursing mothers, those with serious thrombophilic defects, or those requiring concomitant antiplatelet therapy, remain to be established. Therefore, additional studies are needed to optimize the role of NOACs for VTE treatment.
Acknowledgments
The authors thank Professor Robin Roberts for statistical support.
J.I.W. holds the Canada Research Chair (Tier I) in Thrombosis and the Heart and Stroke Foundation J. Fraser Mustard Chair in Cardiovascular Research at McMaster University.
This study was supported by a Doctoral Scholarship from the Canadian Institutes of Health Research (C.H.Y.).
Authorship
Contribution: C.H.Y., P.L.G., and J.I.W. analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: P.L.G. has received honoraria from Pfizer, Bayer, and Leo. J.I.W. has served as a consultant and received honoraria from Bristol-Myers Squibb, Pfizer, Daiichi Sankyo, Bayer, Janssen, Boehringer Ingelheim, and Portola. The remaining author declares no competing financial interests.
Correspondence: Jeffrey I. Weitz, Thrombosis and Atherosclerosis Research Institute, 237 Barton St E, Hamilton, ON, Canada L8L 2X2; e-mail: weitzj@taari.ca.
Appendix
Abbreviations for clinical trials
AMPLIFY, Apixaban for the Initial Management of Pulmonary Embolism and Deep-Vein Thrombosis as First-Line Therapy; AMPLIFY-Extension, Apixaban after the Initial Management of Pulmonary Embolism and Deep Vein Thrombosis with First-Line Therapy–Extended Treatment, EINSTEIN-DVT, Oral, direct Factor Xa inhibitor rivaroxaban in patients with acute symptomatic deep vein thrombosis; EINSTEIN-PE, Oral, direct Factor Xa inhibitor rivaroxaban in patients with acute symptomatic pulmonaryembolism; EINSTEIN-Extension, Once-daily oral rivaroxaban versus placebo in the long-term prevention of recurrent symptomatic venous thromboembolism; Hokusai-VTE, Comparative Investigation of Low Molecular Weight Heparin/Edoxaban Tosylate Versus Low Molecular Weight Heparin/Warfarin in the Treatment of Symptomatic Deep-Vein Blood Clots and/or Lung Blood Clots; RE-COVER I, Efficacy and Safety of Dabigatran Compared with Warfarin for 6 Month Treatment of Acute Symptomatic Venous Thromboembolism; RE-COVER II, Phase III Study Testing Efficacy & Safety of Oral Dabigatran Etexilatevs Warfarin for 6 m Treatment of Acute Symptomatic Venous Thromboembolism; RE-MEDY, A Phase III, Randomized, Multicenter, Double-blind, Parallel-group, Active Controlled Study to Evaluate the Efficacy and Safety of Oral Dabigatran Etexilate Compared with Warfarin for the Secondary Prevention of Venous Thromboembolism; RE-SONATE, Twice-daily Oral Direct Thrombin Inhibitor Dabigatran Etexilate in the Long Term Prevention of Recurrent Symptomatic VTE.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal