Key Points
Twice-weekly oral ixazomib appears tolerable, with no severe neuropathy seen to date, in heavily pretreated multiple myeloma patients.
These phase 1 data suggest clinical activity including 76% stable disease or better, with durable responses and sustained disease control.
Abstract
Ixazomib is the first investigational oral proteasome inhibitor to be studied clinically. In this phase 1 trial, 60 patients with relapsed/refractory multiple myeloma (median of 4 prior lines of therapy; bortezomib, lenalidomide, thalidomide, and carfilzomib/marizomib in 88%, 88%, 62%, and 5%, respectively) received single-agent ixazomib 0.24 to 2.23 mg/m2 (days 1, 4, 8, 11; 21-day cycles). Two dose-limiting toxicities (grade 3 rash; grade 4 thrombocytopenia) occurred at 2.23 mg/m2. The maximum tolerated dose was 2.0 mg/m2, which 40 patients received in 4 expansion cohorts. Patients received a median of 4 cycles (range, 1-39); 18% received ≥12 cycles. Eighty-eight percent had drug-related adverse events, including nausea (42%), thrombocytopenia (42%), fatigue (40%), and rash (40%); drug-related grade ≥3 events included thrombocytopenia (37%) and neutropenia (17%). Grade 1/2 drug-related peripheral neuropathy occurred in 12% (no grade ≥3). Two patients died on the study (both considered unrelated to treatment). The terminal half-life of ixazomib was 3.3 to 7.4 days; plasma exposure increased proportionally with dose (0.48-2.23 mg/m2). Among 55 response-evaluable patients, 15% achieved partial response or better (76% stable disease or better). These findings have informed the subsequent clinical development of ixazomib in multiple myeloma. This trial was registered at www.clinicaltrials.gov as #NCT00932698.
Introduction
The validity of proteasome inhibition as an anticancer strategy has been demonstrated with bortezomib,1 which has been shown to be efficacious in multiple myeloma (MM)2-4 and is approved for the treatment of MM in the United States5 and European Union.6 Recently, the US Food and Drug Administration approved another proteasome inhibitor, carfilzomib, for the treatment of patients with MM who have received ≥2 prior therapies, including bortezomib and an immunomodulatory agent, and have demonstrated disease progression on or within 60 days of the completion of the last therapy.7 Additional new investigational proteasome inhibitors are being developed,1,8 which differ in terms of chemical class, enzyme binding kinetics, and, for some, route of administration, as well as toxicity.1
Among these is the investigational proteasome inhibitor ixazomib, an orally bioavailable, small molecule inhibitor of the 20S proteasome.9 Ixazomib (MLN2238) refers to the biologically active boronic acid form of ixazomib citrate (MLN9708). The drug substance is administered as a stable citrate ester (ixazomib citrate) that, under physiological conditions, rapidly hydrolyses to ixazomib. Ixazomib preferentially binds to and inhibits the chymotrypsin-like site of the 20S proteasome and immunoproteasome, as well as, at higher concentrations, the caspase-like and trypsin-like sites.9 Ixazomib has physiochemical properties distinct from bortezomib. Ixazomib has demonstrated similar selectivity and potency to bortezomib in biochemical and cell-based assays but has a shorter 20S proteasome dissociation half-life.9 Ixazomib has shown in vitro activity and antitumor activity across xenograft models, including in vivo models of MM, some of which were bortezomib resistant.9-12
Ixazomib is the first oral proteasome inhibitor to enter clinical investigation. The early development of single-agent ixazomib in patients with relapsed and/or refractory MM involved 2 similar phase 1 dose-escalation studies investigating different dosing schedules commonly used with bortezomib. This paper and a companion paper by Kumar et al13 report the findings of these clinical studies. Here we report the results of the phase 1 study of twice-weekly oral ixazomib (#NCT00932698), which represents the same dosing schedule used in the phase 3 Assessment of Proteasome Inhibition for Extending Remissions (APEX) study of bortezomib.3
Patients and methods
Patients
All patients were ≥18 years of age and required measurable disease, Eastern Cooperative Oncology Group performance status of 0 to 2, absolute neutrophil count ≥1000/mm3, platelet count ≥75 000/mm3, total bilirubin ≤1.5 × the upper limit of normal, alanine aminotransferase and aspartate aminotransferase ≤ 2.5 × upper limit of normal, and calculated creatinine clearance ≥ 20 mL/minute within 3 days before the first dose. Key exclusion criteria for all patients were grade ≥2 peripheral neuropathy (PN; exclusion criterion used for other studies of proteasome inhibitors3,4,14,15 ); grade >1 diarrhea; and major surgery, serious infection, radiotherapy, or systemic treatment with strong CYP1A2 inhibitors or strong inhibitors/inducers of CYP3A within 14 days or investigational products within 21 days before first dose. No prior exposure to investigational proteasome inhibitors was permitted, except carfilzomib. Patients were excluded for severe or uncontrolled preexisting comorbidities that in the investigator’s opinion could potentially interfere with tolerance of ixazomib, oral absorption, or completion of treatment.
In the dose-escalation cohort, patients had to have relapsed following ≥2 prior lines of therapy that must have included bortezomib, thalidomide or lenalidomide, and corticosteroids, in any combinations. Criteria for the maximum tolerated dose (MTD) expansion cohorts differed only in terms of prior therapies and relapsed or refractory disease status (refractory defined as progressive disease while on therapy or within 60 days of last dose). In the relapsed and refractory cohort, patients were refractory to their most recent prior therapy (any regimen). In the bortezomib-relapsed cohort, patients had relapsed or were refractory following ≥1 prior line (patients could be refractory to any regimen except previous bortezomib therapy), had relapsed following but were not refractory to previous bortezomib therapy, and had not received any other prior proteasome inhibitor. In the proteasome inhibitor-naïve cohort, patients had relapsed or were refractory following ≥1 prior line that must have included thalidomide or lenalidomide and a corticosteroid, but no proteasome inhibitor. In the prior carfilzomib cohort, patients had relapsed or were refractory (to any regimen) following ≥2 prior lines and had previously received carfilzomib. All patients provided written informed consent. Review boards at all participating institutions approved the study, which was conducted according to the provisions of the Declaration of Helsinki, the International Conference on Harmonization, and the Guidelines for Good Clinical Practice.
Study design
This open-label phase 1 study (#NCT00932698) was conducted at 5 sites in the United States, with patients enrolled from September 30, 2009 to June 6, 2012. The primary objectives were to determine the safety profile, tolerability, and MTD of oral twice-weekly ixazomib and to inform the recommended phase 2 dose. Secondary objectives were to characterize plasma pharmacokinetics of ixazomib and to determine overall response rate in patients with relapsed and/or refractory MM and in the expansion cohorts.
Patients received single-agent oral ixazomib on days 1, 4, 8, and 11 of a 21-day cycle, for up to 12 cycles or until disease progression or unacceptable toxicity. Patients who were determined to potentially benefit from prolonged therapy could receive continued treatment beyond 12 cycles. Concomitant corticosteroids at a therapeutic dose (>10 mg prednisone or equivalent per day) were not allowed; however, lower-dose topical or oral steroids were permitted in cases of rash. Dose escalation proceeded via a standard 3 + 3 schema, using a modified Fibonacci dose sequence. Dose-escalation decisions were based on dose-limiting toxicities (DLTs) occurring in cycle 1; the MTD was defined as the highest dose level at which 0 of 6 or 1 of 6 patients experience DLTs during cycle 1. The following were defined as DLTs: grade 4 neutropenia or thrombocytopenia lasting >7 consecutive days or a platelet count <10 000/mm3 at any time; grade 3 neutropenia with infection or thrombocytopenia with clinically significant bleeding; grade 2 PN with pain or grade ≥ 3 PN; any other grade ≥ 3 nonhematologic toxicity, except grade 3 arthralgia/myalgia or brief (<1 week) grade 3 fatigue; a delay of ≥2 weeks in commencing cycle 2 due to lack of adequate recovery of ixazomib-related toxicities; and any other grade ≥2 ixazomib-related toxicities requiring discontinuation in the opinion of the investigator. Once the MTD was established, patients were enrolled to 4 expansion cohorts, as defined previously. Patients from the dose-escalation cohort treated at the MTD and meeting the criteria of one of the MTD expansion cohorts were combined with patients enrolled to that expansion cohort.
Assessments
Adverse events (AEs) were monitored throughout the study. AEs were graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (v3.0). Responses were assessed using the International Myeloma Working Group uniform criteria,16 incorporating minimal response (MR) per the European Group for Blood and Marrow Transplantation criteria.17 Responses were assessed by investigators.
Blood samples for determination of plasma ixazomib for pharmacokinetic analysis (3 mL) were collected predose (within 1 hour) on days 1, 4, and 11 of cycle 1, and day 1 of cycle 2; on days 1 and 11, cycle 1, at 15 and 30 minutes, and 1, 1.5, 2, 4, 8, and 24 hours after dosing (as well as 96 and 168 hours after the day 11 dose). Plasma concentrations of ixazomib were measured using a validated liquid chromatography/tandem mass spectrometry assay.
Statistical analyses
The safety population included all patients who received ≥1 dose of ixazomib. The DLT-evaluable population comprised patients who received all cycle 1 doses of ixazomib and completed cycle 1 or experienced a DLT in cycle 1. The DLT-evaluable population was used for determination of the MTD. The response-evaluable population included all patients who received ≥1 dose of ixazomib, had measurable disease at baseline, and had ≥1postbaseline disease assessment. Pharmacokinetic parameters were calculated using noncompartmental methods (WinNonlin software [v5.3]). All data were summarized using descriptive statistics; no formal comparisons were performed between expansion cohorts.
Results
Patients
Sixty patients were enrolled. Twenty-six patients were enrolled to the dose-escalation cohorts, including 3 patients each at 0.24, 0.48, 0.8, 1.2, and 1.68 mg/m2, 7 patients at 2.0 mg/m2, and 4 patients at 2.23 mg/m2. Forty patients were enrolled to the expansion cohorts, which included 6 from the 2.0 mg/m2 MTD dose-escalation cohort; 20, 12, 6, and 2 were enrolled to the relapsed and refractory, bortezomib-relapsed, proteasome inhibitor-naïve, and prior carfilzomib cohorts, respectively. Patients’ demographics and baseline disease characteristics are summarized in Table 1; patients had received a median of 4 prior lines of therapy, including bortezomib, lenalidomide, thalidomide, and carfilzomib/marizomib in 88%, 88%, 62%, and 5%, respectively. Sixty percent were refractory to their last prior therapy, including 27% who were bortezomib refractory on their last prior therapy.
Patients’ demographics and baseline disease characteristics
| Characteristic . | Dose-escalation cohorts (n = 26) . | Expansion cohorts (n = 40)* . | Total (n = 60) . |
|---|---|---|---|
| Median age, years (range) | 65 (50-83) | 65 (50-86) | 65 (50-86) |
| Male, n (%) | 16 (62) | 19 (48) | 32 (53) |
| Race, n (%) | |||
| White | 22 (85) | 38 (95) | 54 (90) |
| African American | 4 (15) | 1 (3) | 5 (8) |
| Other | 0 | 1 (3) | 1 (2) |
| ECOG performance status, n (%)† | |||
| 0 | 6 (23) | 16 (40) | 19 (32) |
| 1 | 17 (65) | 20 (50) | 35 (58) |
| 2 | 3 (12) | 3 (8) | 5 (8) |
| ISS disease stage, n (%)‡ | |||
| I | 8 (31) | 13 (33) | 20 (33) |
| II | 11 (42) | 13 (33) | 20 (33) |
| III | 7 (27) | 13 (33) | 19 (32) |
| Median creatinine clearance, mL/min (range) | 76.9 (23.5-163.0) | 67.7 (25.7-125.3) | 73.6 (23.5-163.0) |
| Creatinine clearance <50 mL/min, n (%)§ | 7 (27) | 13 (33) | 18 (30) |
| Median time since MM diagnosis, years (range) | 4.7 (1.1-24.3) | 4.9 (1.0-12.6) | 4.8 (1.0-24.3) |
| MM subtype, n (%) | |||
| IgG | 14 (54) | 27 (68) | 38 (63) |
| IgA | 5 (19) | 8 (20) | 11 (18) |
| Λ light chain | 4 (15) | 2 (5) | 6 (10) |
| κ light chain | 3 (12) | 3 (8) | 5 (8) |
| Median number of prior lines of therapy, n (range) | 4 (2-28) | 4 (1-12) | 4 (1-28) |
| Prior therapy included:, n (%) | |||
| Bortezomib | 26 (100) | 33 (83) | 53 (88) |
| Lenalidomide | 23 (88) | 35 (88) | 53 (88) |
| Thalidomide | 16 (62) | 26 (65) | 37 (62) |
| Carfilzomib/marizomib | 1 (4) | 2 (5) | 3 (5) |
| Stem cell transplantation | 16 (62) | 23 (58) | 36 (60) |
| Patients refractory to their last prior therapy, n (%)¶ | 14 (58) | 25 (64) | 34 (60) |
| Bortezomib-refractory, n (%) | 7 (27) | 12 (30) | 16 (27) |
| Cytogenetic abnormalities, n (%) | |||
| del 17 | 4 (16) | 3 (10) | 5 (10) |
| t(4;14) | 1 (4) | 2 (7) | 2 (4) |
| Hyperdiploidy | 3 (12) | 7 (23) | 8 (16) |
| Characteristic . | Dose-escalation cohorts (n = 26) . | Expansion cohorts (n = 40)* . | Total (n = 60) . |
|---|---|---|---|
| Median age, years (range) | 65 (50-83) | 65 (50-86) | 65 (50-86) |
| Male, n (%) | 16 (62) | 19 (48) | 32 (53) |
| Race, n (%) | |||
| White | 22 (85) | 38 (95) | 54 (90) |
| African American | 4 (15) | 1 (3) | 5 (8) |
| Other | 0 | 1 (3) | 1 (2) |
| ECOG performance status, n (%)† | |||
| 0 | 6 (23) | 16 (40) | 19 (32) |
| 1 | 17 (65) | 20 (50) | 35 (58) |
| 2 | 3 (12) | 3 (8) | 5 (8) |
| ISS disease stage, n (%)‡ | |||
| I | 8 (31) | 13 (33) | 20 (33) |
| II | 11 (42) | 13 (33) | 20 (33) |
| III | 7 (27) | 13 (33) | 19 (32) |
| Median creatinine clearance, mL/min (range) | 76.9 (23.5-163.0) | 67.7 (25.7-125.3) | 73.6 (23.5-163.0) |
| Creatinine clearance <50 mL/min, n (%)§ | 7 (27) | 13 (33) | 18 (30) |
| Median time since MM diagnosis, years (range) | 4.7 (1.1-24.3) | 4.9 (1.0-12.6) | 4.8 (1.0-24.3) |
| MM subtype, n (%) | |||
| IgG | 14 (54) | 27 (68) | 38 (63) |
| IgA | 5 (19) | 8 (20) | 11 (18) |
| Λ light chain | 4 (15) | 2 (5) | 6 (10) |
| κ light chain | 3 (12) | 3 (8) | 5 (8) |
| Median number of prior lines of therapy, n (range) | 4 (2-28) | 4 (1-12) | 4 (1-28) |
| Prior therapy included:, n (%) | |||
| Bortezomib | 26 (100) | 33 (83) | 53 (88) |
| Lenalidomide | 23 (88) | 35 (88) | 53 (88) |
| Thalidomide | 16 (62) | 26 (65) | 37 (62) |
| Carfilzomib/marizomib | 1 (4) | 2 (5) | 3 (5) |
| Stem cell transplantation | 16 (62) | 23 (58) | 36 (60) |
| Patients refractory to their last prior therapy, n (%)¶ | 14 (58) | 25 (64) | 34 (60) |
| Bortezomib-refractory, n (%) | 7 (27) | 12 (30) | 16 (27) |
| Cytogenetic abnormalities, n (%) | |||
| del 17 | 4 (16) | 3 (10) | 5 (10) |
| t(4;14) | 1 (4) | 2 (7) | 2 (4) |
| Hyperdiploidy | 3 (12) | 7 (23) | 8 (16) |
ECOG, Eastern Cooperative Oncology Group; ISS, International Staging System.
Includes 6 patients treated at 2.0 mg/m2 in the dose-escalation cohorts.
Not done for 1 patient in the expansion cohorts.
Unknown in 1 patient in the expansion cohorts.
Three patients, 2 in the dose-escalation cohorts and 2 in the expansion cohorts (including 1 treated at 2.0 mg/m2 in the dose-escalation cohort) had creatinine clearance of 21 to 30 mL/minute.
Missing for 2 patients in the dose-escalation cohorts, 1 patient in the expansion cohorts, and 3 patients overall.
DLTs and MTD
Of 26 patients in the dose-escalation cohorts, 24 were included in the DLT-evaluable population. Two patients, treated at 2.0 and 2.23 mg/m2, were excluded. One patient treated at 2.23 mg/m2 reported an AE that met the formal definition of a DLT. The patient experienced grade 3 macular erythematous rash; ixazomib dosing was held and the dose was reduced. Following treatment with oral antihistamine, topical corticosteroid cream, and low-dose oral prednisone taper, the rash resolved, and the patient continued at a lower dose. An additional patient treated at 2.23 mg/m2 experienced grade 4 thrombocytopenia with a platelet count of 10 000/mm3 that required a platelet transfusion. This AE did not meet the formal definition of a DLT (platelet count was not <10 000/mm3; duration was not >7 days) but was conservatively counted as a DLT by the investigator and sponsor. Both DLTs were transient and clinically manageable with standard medical interventions. After discussions with the principal investigators and as allowed per protocol, an intermediate dose of 2.0 mg/m2 was evaluated. As no DLTs were observed, the MTD of ixazomib on a twice-weekly schedule was determined to be 2.0 mg/m2; this represents the approximate equivalent to a fixed dose of 3.7 mg, based on population pharmacokinetic analysis.18,19
Treatment exposure and safety profile
Patients received a median of 4 cycles (range, 1-39) of treatment; 24 (40%) patients received ≥6 cycles and 11 (18%) received ≥12 cycles. At data cutoff (March 11, 2013), 6 (10%) patients remained on treatment having received 7, 17, 30, 34, 37, and 39 cycles, respectively. Fifty-four patients discontinued study treatment for the primary reasons of progressive disease in 37 (62%), patient withdrawal in 9 (15%), AEs in 7 (12%), and death in 1 (2%). Mean ixazomib dose intensity was 5.5 mg/m2 per cycle (range, 0.77-8.07); the mean ranged from 0.8 to 5.9 mg/m2 per cycle in the 0.24 and 2.23 mg/m2 dose-escalation cohorts, respectively. Across the expansion cohorts, at 2.0 mg/m2, mean dose intensity was 6.4 mg/m2 per cycle, which, with a planned maximum dose of 8.0 mg/m2 per cycle, represents a relative dose intensity of 80%. Among all patients, estimated dosing compliance was 94% (mean of 27.7 doses received in a mean of 7.4 cycles [29.6 planned doses], accounted for by, for example, doses being held due to AEs, missed, and other reasons).
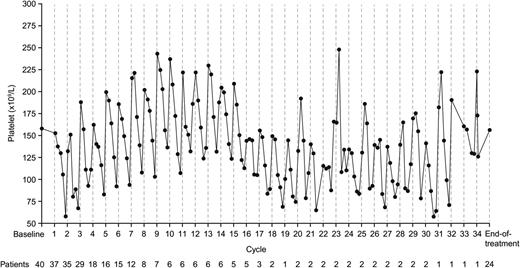
All patients were included in the safety population. The safety profile and most common drug-related AEs are summarized in Table 2. The most common drug-related grade ≥3 AEs are shown in Table 3. All-cause and drug-related AEs are summarized by grade in supplemental Table 1. As shown in supplemental Table 2, the safety profile and common drug-related AEs generally did not appear substantially different in the small population of patients with renal impairment (creatinine clearance <50 mL/min). There were no clinically significant drug-related cardiac events reported. Thrombocytopenia was the most common drug-related grade 4 AE, occurring in 14 (23%) patients. Other drug-related grade 4 AEs were hyperuricemia and neutropenia, each seen in 1 (2%) patient. Thrombocytopenia appeared transient, often self-limiting, and cyclical. Figure 1 shows mean platelet count among all patients treated at 2.0 mg/m2. In total, 15 (25%) patients required platelet transfusions due to thrombocytopenia, primarily for grade 4 events (n = 3 grade 3-related events), which were administered per standard guidelines.
Summary of the ixazomib safety profile plus the most common (≥10% of patients overall) drug-related AEs, overall and within the dose-escalation and expansion cohorts
| AE, n (%) . | Dose-escalation cohorts (n = 26) . | Expansion cohorts (n = 40)* . | Total (n = 60) . |
|---|---|---|---|
| Any AE | 26 (100) | 40 (100) | 60 (100) |
| Any drug-related AE | 21 (81) | 38 (95) | 53 (88) |
| Any grade ≥3 AE | 19 (73) | 33 (83) | 47 (78) |
| Any drug-related grade ≥3 AE | 13 (50) | 27 (68) | 37 (62) |
| Any grade 4 AE | 7 (27) | 19 (48) | 23 (38) |
| Any drug-related grade 4 AE | 5 (19) | 13 (33) | 16 (27) |
| Any dose reduction due to AEs | 7 (27) | 21 (53) | 27 (45) |
| Any SAE† | 11 (42) | 24 (60) | 31 (52) |
| Common drug-related AEs | |||
| Nausea | 10 (38) | 18 (45) | 25 (42) |
| Thrombocytopenia | 9 (35) | 19 (48) | 25 (42) |
| Fatigue | 9 (35) | 16 (40) | 24 (40) |
| Skin and SC tissue disorders‡ | 11 (42) | 15 (38) | 24 (40) |
| Vomiting | 8 (31) | 8 (20) | 15 (25) |
| Diarrhea | 8 (31) | 8 (20) | 14 (23) |
| Pyrexia | 4 (15) | 8 (20) | 12 (20) |
| Neutropenia | 5 (19) | 7 (18) | 11 (18) |
| Peripheral neuropathy§ | 3 (12) | 5 (13) | 7 (12) |
| Chills | 2 (8) | 4 (10) | 6 (10) |
| AE, n (%) . | Dose-escalation cohorts (n = 26) . | Expansion cohorts (n = 40)* . | Total (n = 60) . |
|---|---|---|---|
| Any AE | 26 (100) | 40 (100) | 60 (100) |
| Any drug-related AE | 21 (81) | 38 (95) | 53 (88) |
| Any grade ≥3 AE | 19 (73) | 33 (83) | 47 (78) |
| Any drug-related grade ≥3 AE | 13 (50) | 27 (68) | 37 (62) |
| Any grade 4 AE | 7 (27) | 19 (48) | 23 (38) |
| Any drug-related grade 4 AE | 5 (19) | 13 (33) | 16 (27) |
| Any dose reduction due to AEs | 7 (27) | 21 (53) | 27 (45) |
| Any SAE† | 11 (42) | 24 (60) | 31 (52) |
| Common drug-related AEs | |||
| Nausea | 10 (38) | 18 (45) | 25 (42) |
| Thrombocytopenia | 9 (35) | 19 (48) | 25 (42) |
| Fatigue | 9 (35) | 16 (40) | 24 (40) |
| Skin and SC tissue disorders‡ | 11 (42) | 15 (38) | 24 (40) |
| Vomiting | 8 (31) | 8 (20) | 15 (25) |
| Diarrhea | 8 (31) | 8 (20) | 14 (23) |
| Pyrexia | 4 (15) | 8 (20) | 12 (20) |
| Neutropenia | 5 (19) | 7 (18) | 11 (18) |
| Peripheral neuropathy§ | 3 (12) | 5 (13) | 7 (12) |
| Chills | 2 (8) | 4 (10) | 6 (10) |
SC, subcutaneous.
Includes 6 patients treated at 2.0 mg/m2 in the dose-escalation cohorts.
Common drug-related SAEs included thrombocytopenia in 5 (8%) patients, pyrexia in 4 (7%), abdominal pain in 3 (5%), and dehydration and orthostatic hypotension each in 2 (3%); all other drug-related SAEs were reported in only 1 patient each.
MedDRA System Organ Class, includes rash macular (n = 11, 18%), rash (n = 6, 10%), erythema, rash maculo-papular, rash papular (each n = 4, 7%), pruritis (n = 3, 5%), dry skin, rash pruritic, skin exfoliation (each n = 2, 3%), erythema multiforme, hyperhidrosis, petechiae, photodermatosis, rash erythematous, skin discoloration, skin hyperpigmentation, skin lesion, swelling face, urticaria, and vasculitic rash (each n = 1, 2%). Patients could have reported >1 AE.
High-level term, peripheral neuropathies NEC includes neuropathy peripheral and peripheral sensory neuropathy.
Most common (≥3% of patients overall) drug-related grade ≥3 AEs, overall and within the dose-escalation and expansion cohorts
| AE (MedDRA preferred term), n (%) . | Dose-escalation cohorts (n = 26) . | Expansion cohorts (n = 40)* . | Total (n = 60) . |
|---|---|---|---|
| Thrombocytopenia | 7 (27) | 18 (45) | 22 (37) |
| Neutropenia | 4 (15) | 7 (18) | 10 (17) |
| Skin and SC tissue disorders† | 1 (4) | 4 (10) | 5 (8) |
| Fatigue | 0 | 4 (10) | 4 (7) |
| Lymphopenia | 0 | 3 (8) | 3 (5) |
| Abdominal pain | 1 (4) | 1 (3) | 2 (3) |
| Hypophosphatemia | 2 (8) | 0 | 2 (3) |
| Leukopenia | 0 | 2 (5) | 2 (3) |
| Orthostatic hypotension | 1 (4) | 1 (3) | 2 (3) |
| WBC count decreased | 1 (4) | 1 (3) | 2 (3) |
| AE (MedDRA preferred term), n (%) . | Dose-escalation cohorts (n = 26) . | Expansion cohorts (n = 40)* . | Total (n = 60) . |
|---|---|---|---|
| Thrombocytopenia | 7 (27) | 18 (45) | 22 (37) |
| Neutropenia | 4 (15) | 7 (18) | 10 (17) |
| Skin and SC tissue disorders† | 1 (4) | 4 (10) | 5 (8) |
| Fatigue | 0 | 4 (10) | 4 (7) |
| Lymphopenia | 0 | 3 (8) | 3 (5) |
| Abdominal pain | 1 (4) | 1 (3) | 2 (3) |
| Hypophosphatemia | 2 (8) | 0 | 2 (3) |
| Leukopenia | 0 | 2 (5) | 2 (3) |
| Orthostatic hypotension | 1 (4) | 1 (3) | 2 (3) |
| WBC count decreased | 1 (4) | 1 (3) | 2 (3) |
WBC, white blood cell.
Includes 6 patients treated at 2.0 mg/m2 in the dose-escalation cohorts.
MedDRA System Organ Class includes rash macular (n = 3, 5%), rash, and rash papular (each n = 1, 2%).
Mean platelet count among patients treated at the MTD of 2.0 mg/m2. Platelet count decreased with each dose during a cycle, reaching a nadir by approximately day 15, with recovery by the planned beginning of the subsequent cycle.
Mean platelet count among patients treated at the MTD of 2.0 mg/m2. Platelet count decreased with each dose during a cycle, reaching a nadir by approximately day 15, with recovery by the planned beginning of the subsequent cycle.
Drug-related AEs in the skin and subcutaneous tissue disorders MedDRA System Organ Class were reported in 24 (40%) patients (Table 2), including macular rash (18%), rash (10%), erythema, rash maculo-papular, and rash papular (each 7%). The rash presented with various clinical descriptions, could be localized or widespread, and was most commonly reported as appearing on the chest, abdomen, and back, and occasionally on the neck, face, arms, hands, and thighs. Two patients had confounding factors regarding causality of rash; there was no clear association. Median time to onset of rash was 2 cycles (range, 1-10). Rash AEs were transient, often self-limiting, and typically grade 1 or 2 in severity. Drug-related rash AEs were shown to be generally manageable using standard symptomatic measures such as antihistamines or corticosteroids (oral or topical), which were also used prophylactically as needed in subsequent cycles. Data (on file) suggest rash was a dose-dependent effect.
Treatment-emergent PN was reported in 10 (17%) patients, with drug-related PN reported in 7 (12%). There was no grade ≥3 PN reported. Among 7 patients with drug-related PN, 3 had grade 1 PN at baseline, with worsening to grade 2 in cycle 2 (0.8 mg/m2 cohort), worsening from baseline but remaining grade 1 in cycle 3 (1.68 mg/m2 cohort), and worsening to grade 2 in cycle 3 (relapsed and refractory cohort). The remaining 4 patients in the relapsed and refractory (n = 1) and bortezomib-relapsed cohort (n = 3) had grade 1 PN in cycle 4, 3, and 4, and grade 2 in cycle 13, respectively.
AEs resulting in ixazomib dose reductions included thrombocytopenia (n = 13, 22%), rash (n = 7, 12%), and neutropenia (n = 3, 5%). Eight (13%) patients had AEs resulting in treatment discontinuation. One patient in the 2.0 mg/m2 dose-escalation cohort had spinal cord compression due to progressive disease in cycle 1, assessed as unrelated by the investigator, which was also a serious AE (SAE). Five patients in the relapsed and refractory cohort discontinued due to drug-related thrombocytopenia in cycle 1; drug-related fall in cycle 3 (SAE); drug-related nausea and fatigue plus PN assessed as unrelated by the investigator in cycle 5; and pneumonia assessed as unrelated by the investigator in cycle 2 (SAE). Another patient in this cohort discontinued for bone pain due to progressive disease in cycle 3. One patient in the bortezomib-relapsed cohort had drug-related pulmonary hypertension (SAE) in cycle 1. One patient in the proteasome inhibitor-naïve cohort had drug-related pruritic rash in cycle 4.
Two patients died on study. One patient with atrial fibrillation and a history of syncope and orthostatic hypotension, who was treated with ixazomib 0.8 mg/m2, died at the end of cycle 7, 12 days after the last dose of ixazomib, due to an undiagnosed cardiac disorder, reported by the investigator as unrelated to ixazomib. One patient in the relapsed and refractory cohort (ixazomib 2.0 mg/m2) died due to progressive disease, reported by the investigator as unrelated to ixazomib, at the end of cycle 3, 20 days after the last dose of ixazomib.
Pharmacokinetics
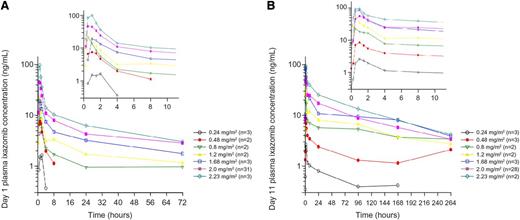
Ixazomib was rapidly absorbed; median Tmax was 1 hour. After day 11 dosing, ixazomib terminal half-life was 3.3 to 7.4 days, and the accumulation ratio (day 11 AUC0-72/day 1 AUC0-72) was 3.35. Ixazomib plasma exposure increased proportionally with increasing ixazomib dose from 0.48 to 2.23 mg/m2 (actual administered dose: 0.8-4.8 mg; Figure 2). Exposures were similar across various expansion cohorts at the MTD; thus, pooled pharmacokinetic data for patients treated at 2.0 mg/m2 are presented (Table 4).
Pharmacokinetic profiles of ixazomib. Mean plasma concentration-time profiles of ixazomib on (A) day 1 and (B) day 11.
Pharmacokinetic profiles of ixazomib. Mean plasma concentration-time profiles of ixazomib on (A) day 1 and (B) day 11.
Geometric mean (%CV) ixazomib plasma pharmacokinetic parameters on days 1 and 11 of cycle 1
| Parameter . | Ixazomib dose, mg/m2 . | ||||||
|---|---|---|---|---|---|---|---|
| 0.24 . | 0.48 . | 0.8 . | 1.2 . | 1.68 . | 2 . | 2.23 . | |
| Day 1 | |||||||
| N | 3 | 2 | 2 | 2 | 3 | 31* | 3 |
| Tmax, hours† | 1 (0.567–2) | 0.5, 1.5 | 0.55, 1 | 0.5, 1.05 | 1 (1–2.02) | 0.65 (0.25–3.97) | 1 (0.717–1.08) |
| Cmax, ng/mL | 2.10 (19) | 8.38, 12 | 14.2, 30.2 | 11.9, 46.1 | 19.3 (48) | 49.0 (66) | 105 (57) |
| AUC0–72, hours × ng/mL | NC | NC | 80, 138 | 94.1, 224 | 246 (25) | 379 (40) | 383, 450 |
| DN Cmax, ng/mL/mg | 4.86 (9) | 10.5, 13.3 | 9.47, 20.1 | 4.96, 15.9 | 6.22 (53) | 13.1 (68) | 25.8 (78) |
| DN AUC0–72, hours × ng/mL/mg | NC | NC | 53.3, 92 | 39.2, 77.2 | 79.3 (30) | 102 (43) | 81.5, 100 |
| Day 11 | |||||||
| N | 3 | 3 | 2 | 2 | 3 | 28‡ | 2 |
| Tmax, hours† | 1.1 (1.05–4.07) | 1 (0.5–1.17) | 0.55, 2 | 0.5, 0.5 | 1 (0.567–1.05) | 1 (0.5–23.6) | 0.533, 1.13 |
| Cmax, ng/mL | 2.50 (53) | 7.78 (63) | 20.9, 42.4 | 46.5, 66.5 | 86.0 (71) | 60.5 (51) | 75.9, 135 |
| AUC0–72, hours × ng/mL | 55.0 (27) | 156 (65) | 428, 488 | 605 | 658, 959 | 1060 (68) | 1810, 2020 |
| DN Cmax, ng/mL/mg | 5.80 (56) | 9.35 (56) | 13.9, 28.3 | 19.4, 22.9 | 27.8 (68) | 16.4 (53) | 16.1, 30 |
| DN AUC0–72, hours × ng/mL/mg | 128 (37) | 188 (58) | 285, 325 | 252 | 206, 331 | 287 (63) | 385, 449 |
| t1/2, hours | NC | 135 | NC | 125, 128 | 128 (20) | 116 (25) | 86.7, 98.7 |
| Accumulation ratio | NC | NC | 3.11, 6.1 | 6.43 | 3.06, 3.49 | 3.09 (55) | 4.5, 4.72 |
| Parameter . | Ixazomib dose, mg/m2 . | ||||||
|---|---|---|---|---|---|---|---|
| 0.24 . | 0.48 . | 0.8 . | 1.2 . | 1.68 . | 2 . | 2.23 . | |
| Day 1 | |||||||
| N | 3 | 2 | 2 | 2 | 3 | 31* | 3 |
| Tmax, hours† | 1 (0.567–2) | 0.5, 1.5 | 0.55, 1 | 0.5, 1.05 | 1 (1–2.02) | 0.65 (0.25–3.97) | 1 (0.717–1.08) |
| Cmax, ng/mL | 2.10 (19) | 8.38, 12 | 14.2, 30.2 | 11.9, 46.1 | 19.3 (48) | 49.0 (66) | 105 (57) |
| AUC0–72, hours × ng/mL | NC | NC | 80, 138 | 94.1, 224 | 246 (25) | 379 (40) | 383, 450 |
| DN Cmax, ng/mL/mg | 4.86 (9) | 10.5, 13.3 | 9.47, 20.1 | 4.96, 15.9 | 6.22 (53) | 13.1 (68) | 25.8 (78) |
| DN AUC0–72, hours × ng/mL/mg | NC | NC | 53.3, 92 | 39.2, 77.2 | 79.3 (30) | 102 (43) | 81.5, 100 |
| Day 11 | |||||||
| N | 3 | 3 | 2 | 2 | 3 | 28‡ | 2 |
| Tmax, hours† | 1.1 (1.05–4.07) | 1 (0.5–1.17) | 0.55, 2 | 0.5, 0.5 | 1 (0.567–1.05) | 1 (0.5–23.6) | 0.533, 1.13 |
| Cmax, ng/mL | 2.50 (53) | 7.78 (63) | 20.9, 42.4 | 46.5, 66.5 | 86.0 (71) | 60.5 (51) | 75.9, 135 |
| AUC0–72, hours × ng/mL | 55.0 (27) | 156 (65) | 428, 488 | 605 | 658, 959 | 1060 (68) | 1810, 2020 |
| DN Cmax, ng/mL/mg | 5.80 (56) | 9.35 (56) | 13.9, 28.3 | 19.4, 22.9 | 27.8 (68) | 16.4 (53) | 16.1, 30 |
| DN AUC0–72, hours × ng/mL/mg | 128 (37) | 188 (58) | 285, 325 | 252 | 206, 331 | 287 (63) | 385, 449 |
| t1/2, hours | NC | 135 | NC | 125, 128 | 128 (20) | 116 (25) | 86.7, 98.7 |
| Accumulation ratio | NC | NC | 3.11, 6.1 | 6.43 | 3.06, 3.49 | 3.09 (55) | 4.5, 4.72 |
AUC0-72, area under the ixazomib plasma concentration vs time curve from time zero to 72 hours after dose; Cmax, maximum observed plasma concentration; DN, dose-normalized; NC, not calculated; t1/2, terminal half-life; Tmax, time of Cmax. Individual values are reported if N < 3.
N = 27 for AUC0-72 and DN AUC0-72.
Values are median and range.
N = 27 for AUC0-72 and DN AUC0-72, 24 for t1/2, and 22 for accumulation ratio.
Response to treatment
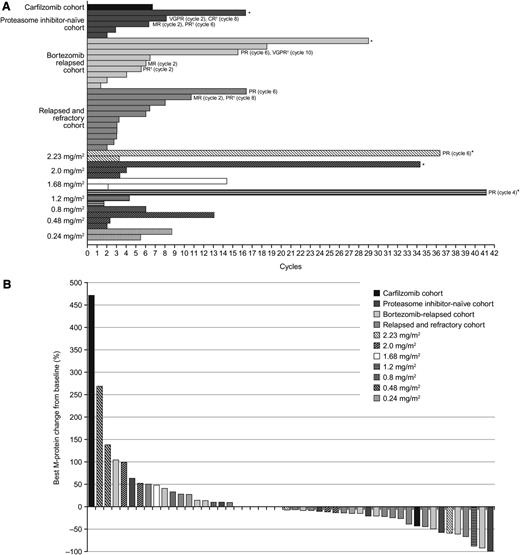
Fifty-five patients were evaluable for response; 5 patients were excluded due to absence of measurable disease at baseline by central laboratory assessment (n = 3) or ≥1 postbaseline disease assessment (n = 2). By investigator assessment, rate of partial response or better was 15% (95% confidence interval: 6%, 27%). Best confirmed or unconfirmed responses included 1 (2%) complete response (CR) in a patient in the proteasome inhibitor-naïve expansion cohort. The patient achieved a very good partial response (VGPR) after 2 cycles, which improved to CR at the end of treatment (cycle 8); the response is ongoing, with a duration of disease control (VGPR or better) of 5.6 months (Figure 3A). One (2%) patient in the bortezomib-relapsed cohort had a VGPR. The patient had an MR after 4 cycles, which improved to partial response (PR) after 6 cycles and to VGPR after 10 cycles; the patient progressed after a duration of disease control of 10.7 months. A further 6 (11%) patients had PRs (1 at 1.2 mg/m2, maintained through 39 cycles to date; 1 at 2.23 mg/m2, maintained through 37 cycles to date; 2 in the relapsed and refractory cohort; 1 in the bortezomib-relapsed cohort; 1 in the proteasome inhibitor-naïve cohort, maintained through 7 cycles to date); time to first response in these 6 patients was 1.6 to 4.4 months, and duration of disease control was 3.8 to 28.3+ months.
Ixazomib treatment duration and response. (A) Duration of stable disease or better and best response to treatment in patients achieving stable disease or better (*patient ongoing on treatment). (B) Individual best M-protein responses (% change from baseline) to ixazomib.
Ixazomib treatment duration and response. (A) Duration of stable disease or better and best response to treatment in patients achieving stable disease or better (*patient ongoing on treatment). (B) Individual best M-protein responses (% change from baseline) to ixazomib.
Additionally, there was 1 (2%) MR in a patient in the bortezomib-relapsed cohort (duration of disease control: 4.2 months). A further 33 (60%) patients achieved stable disease, including 14 of 21 (67%) in the dose-escalation cohorts and 21 of 39 (54%) in the expansion cohorts; the overall rate of stable disease or better was 76% (n = 42). Among these 33 patients, duration of disease control ranged from 0.9 to 23.7+ months (Figure 3A). Best M-protein responses in evaluable patients are shown in Figure 3B.
Discussion
This study is one of the first reports in the literature of the investigational proteasome inhibitor ixazomib in MM patients, which is the first oral proteasome inhibitor to be investigated in the clinic. It provides important findings on the safety profile and pharmacokinetics of ixazomib, as well as a preliminary signal of activity in this setting. The study established the MTD for twice-weekly oral ixazomib as 2.0 mg/m2. The emerging safety profile indicates this dose and schedule can lead to AEs that are generally manageable and reversible with supportive care or dose reductions and, to date, is associated with limited PN in heavily pretreated MM patients, including patients with relapsed and refractory disease previously treated with bortezomib and an immunomodulatory drug. These data also indicate clinical activity in heavily pretreated relapsed and/or refractory MM patients; durable responses and disease control were seen, including in patients previously exposed to proteasome inhibitors.
The only formal DLT seen was grade 3 rash in a patient treated at 2.23 mg/m2, the dose level above the MTD; an additional patient treated at 2.23 mg/m2 experienced grade 4 thrombocytopenia that was considered a DLT despite not meeting the formal definition. These toxicities were readily manageable, as described below. Other studies of oral ixazomib in MM and primary systemic amyloidosis (AL), using twice-weekly and weekly schedules, have reported similar DLTs, including rash, hematologic toxicities, and gastrointestinal events.20,21 Notably, the MTD of twice-weekly ixazomib, of 2.0 mg/m2, is lower than that for weekly oral ixazomib (2.97 mg/m2) in the parallel phase 1 dose-escalation study in a similar MM patient population13 ; however, other studies of weekly oral ixazomib in MM and AL have determined the MTD or recommended phase 2/3 dose as 2.23 mg/m2,20,21 or the fixed-dose equivalent of 4.0 mg based on population pharmacokinetic analysis.18,19
As suggested by the lengthy duration of treatment in a notable proportion of patients—including ≥12 cycles in 18% and >2 years in 3 patients—twice-weekly oral ixazomib was generally well tolerated in this patient population. Common all-grade and grade ≥3 toxicities included fatigue, gastrointestinal toxicities, thrombocytopenia, and rash; AEs were generally manageable with standard concomitant medications. This safety profile reflects some aspects of the safety profile of bortezomib2,3 and carfilzomib,14,15 but also differs in some respects. The rate of drug-related grade ≥3 thrombocytopenia with ixazomib was 37%, similar to the 30% rate of grade 3/4 thrombocytopenia seen with bortezomib in the phase 3 APEX study in proteasome inhibitor-naïve relapsed/refractory MM.22 In the present study, thrombocytopenia was managed with platelet transfusions where required. Additionally, reductions in mean platelet count were transient and cyclical in nature; this characteristic appears common to proteasome inhibitors in patients with MM, having been previously reported with bortezomib22,23 and carfilzomib.24 This may be an effect of the mechanism of action of proteasome inhibition on platelet budding.22,23
Various AEs of rash were common with ixazomib, with 40% of patients reporting drug-related AEs with in the skin and subcutaneous tissue disorders MedDRA System Organ Class, including 8% with grade ≥ 3 events. Rash AEs were generally manageable with antihistamines or low-dose topical or oral corticosteroids and reversible in the majority of instances following use of concomitant medication, dose reduction, or, in some cases, spontaneously. Twice-weekly oral ixazomib was associated with a low rate of PN and no grade ≥ 3 PN in this study, which differs notably from the established safety profile of intravenously administered twice-weekly bortezomib, for which PN is a well-known and comprehensively characterized toxicity.25,26 The rates of PN with ixazomib in this study are similar to the low rates reported with carfilzomib in relapsed/refractory MM patients.14,15
Pharmacokinetic data suggested rapid absorption and a dose-proportional increase in plasma exposure of ixazomib, which was similar across the expansion cohorts. The accumulation ratio was consistent with the twice-weekly ixazomib dosing regimen and the observed terminal half-life.
The responses seen with twice-weekly oral single-agent ixazomib indicate clinical activity in this heavily pretreated patient population. A total of 9 patients achieved MR or better, including 8 (15%) who achieved PR or better. One proteasome-naïve patient achieved a CR, 1 patient who had relapsed following prior bortezomib achieved a VGPR, and 6 patients had PRs, including 5 who had previously been exposed to bortezomib, 2 of whom were in the relapsed and refractory cohort. The duration of responses and stable disease was also notable, with almost one-fifth of patients remaining on treatment for ≥12 cycles (∼9 months). These findings further suggest the feasibility and tolerability of prolonged therapy with this oral proteasome inhibitor. The response rate appears similar in the context of data with other proteasome inhibitors in heavily pretreated populations; in the phase 2 SUMMIT (Study of Uncontrolled Multiple Myeloma Managed with Proteasome Inhibition Therapy) study in proteasome inhibitor-naïve patients,2 bortezomib resulted in a 27% rate of PR or better, whereas in the phase 2 PX-171-003-A1 study in bortezomib-exposed patients (including 73% bortezomib-refractory patients), the response rate with carfilzomib was 24%.14
In conclusion, the findings of this and the parallel phase 1 study of weekly oral ixazomib13 have informed the subsequent clinical development of ixazomib in MM, with phase 3 studies now in progress. Specifically, ongoing randomized, double-blind, multicenter studies are investigating weekly dosing of ixazomib 4.0 mg (vs placebo) plus lenalidomide-dexamethasone in relapsed or relapsed and refractory MM patients following 1 to 3 prior lines of therapy (#NCT01564537) and in previously untreated transplant-ineligible MM patients (#NCT01850524), and the same dose and schedule of ixazomib is being investigated in another phase 3 study, in combination with dexamethasone vs physician’s choice plus dexamethasone in patients with relapsed or refractory AL after 1 or 2 prior therapies (#NCT01659658). Other ongoing early-phase studies of ixazomib include investigations of both weekly (#NCT01217957) and twice-weekly (#NCT01383928) oral ixazomib in combination with lenalidomide-dexamethasone in previously untreated MM patients in phase 1/2 studies,27 and investigation of weekly and twice-weekly oral ixazomib in combination with melphalan-prednisone in previously untreated transplant-ineligible MM patients as part of a phase 1/2 study (#NCT01335685).28
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all patients included in this study and their families, as well as all other physicians, research nurses, study coordinators, and research staff participating in the study. The authors acknowledge Steve Hill, a medical writer with FireKite, part of KnowledgePoint360, an Ashfield Company, for writing support during the development of this manuscript, which was funded by Millennium: The Takeda Oncology Company.
Authorship
Contribution: P.G.R., R.B., M.W., A.J.J., J.P.L., R.D.H., M.T., D.B., N.G., A.-M.H., and S.L. designed the research; P.G.R., R.B., M.W., A.J.J., J.P.L., R.D.H., M.T., D.B., N.G., A.D.B., and S.L. performed the research; P.G.R., R.B., M.W., A.J.J., J.P.L., R.D.H., M.T., D.B., G.L., J.Y., N.G., and S.L. collected data; D.B., G.L., J.Y., and N.G. performed statistical analysis; P.G.R., R.B., M.W., A.J.J., J.P.L., R.D.H., M.T., D.V., G.L., J.Y., N.G., A.-M.H., and S.L. analyzed and interpreted data; P.G.R., D.B., J.Y., and N.G. wrote the manuscript; and all authors reviewed the draft manuscript and approved the final version for submission.
Conflict-of-interest disclosure: P.G.R. is an advisory committee member for Celgene, Janssen Pharmaceuticals, and Millennium: The Takeda Oncology Company, and has received research funding from Celgene and Millennium: The Takeda Oncology Company. R.B. has received research funding from Millennium: The Takeda Oncology Company, Celgene, Novartis, Bristol-Myers Squibb, Sanofi, and Karyopharm. M.W. has received research funding from Millennium: The Takeda Oncology Company. A.J.J. is a consultant for and has received honoraria from Millennium: The Takeda Oncology Company, Celgene, Centocor Ortho Biotech, Exelixis, and Bristol-Myers Squibb, is a consultant for Onyx Pharmaceuticals, and is an advisory committee member for Millennium: The Takeda Oncology Company, Centocor Ortho Biotech, Exelixis, Bristol-Myers Squibb, Celgene, and Onyx Pharmaceuticals. J.P.L. has received research funding from Novartis and Onyx. R.D.H. has received research funding from Millennium: The Takeda Oncology Company, Celgene Corporation, Onyx Pharmaceuticals, and Novartis. D.B., G.L., N.G., A.D.B., and A.-M.H. are employees of Takeda Pharmaceuticals International Co. J.Y. was an employee of Takeda Pharmaceuticals International Co. at the time of this study. S.L. has a consultant/advisory role with Millennium: The Takeda Oncology Company, Celgene, Bristol-Myers Squibb, Novartis, Onyx, and Merck. The remaining author declares no competing financial interests.
Current address of A.J.J. is University of Chicago Medical Center, Chicago, IL.
Correspondence: Paul G. Richardson, Dana-Farber Cancer Institute, 44 Binney St, Dana 1B02, Boston, MA 02115; e-mail: paul_richardson@dfci.harvard.edu.