In this issue of Blood, O’Connor and colleagues describe infection-related mortality on the United Kingdom Childhood Acute Lymphoblastic Leukaemia Randomised Trial 2003 (UKALL 2003) for children with newly diagnosed acute lymphoblastic leukemia, reporting a 5-year cumulative incidence of 2.4%, with Down syndrome (DS) being the factor most predictive of increased risk.1
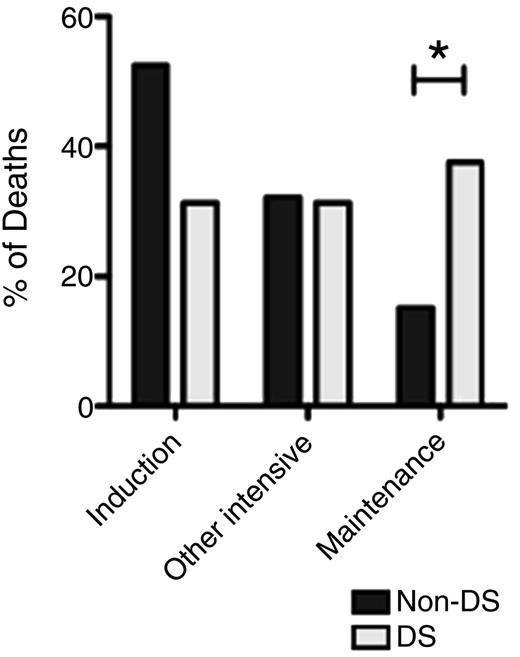
Timing of IRM in DS and non-DS patients (*P < .048). See Figure 2A in the article by O'Connor et al that begins on page 1056.
Timing of IRM in DS and non-DS patients (*P < .048). See Figure 2A in the article by O'Connor et al that begins on page 1056.
Overall survival in childhood ALL has steadily improved during the last several decades, to a current rate of more than 90%.2-4 These dramatic gains in survival, largely attributable to lower relapse rates, have been accompanied by increasing attention to the importance of reducing treatment-related mortality (TRM), which is mainly a result of infectious causes. For intermediate- and high-risk ALL, the progressive intensification of chemotherapy on modern ALL treatment regimens has reduced relapses, but with the unavoidable consequence of a greater risk for TRM. For low-risk ALL, TRM is also a concern, as outstanding survival rates mean the number of deaths caused by TRM now equals the number of deaths resulting from relapse.2 The highly intensive regimens required for treatment of pediatric acute myeloid leukemia have generated attention to the study of TRM,5 but investigations of similar scale and depth are lacking in ALL. Data from large cooperative group trials such as the present study are important to characterize the problem and suggest approaches to reduce it.
O’Connor and colleagues report on the largest infection-related mortality (IRM) cohort in pediatric ALL to date, a retrospective analysis of UKALL 2003, a recently completed trial for the treatment of newly diagnosed ALL in children aged 1 to 24 years, which accrued 3126 subjects between 2003 and 2011.2 Patients were stratified to standard-, intermediate-, or high-risk treatment on the basis of clinical features at presentation, cytogenetics, and response to induction therapy. In total, there were 75 cases of IRM, which constituted 2.4% of the eligible patients, 30.1% of the 249 deaths overall, and 64% of the 117 treatment-related deaths. DS was the factor most significantly associated with IRM, with an odds ratio of 12.08, followed by higher-intensity treatment regimen and National Cancer Institute high-risk status (age at diagnosis >10 years and/or initial white blood count >50 × 109/L). In terms of timing, IRM occurred more often during induction than during any other treatment phase in the cohort overall. Of note, however, IRM in children with DS was more evenly distributed across all treatment phases, with a significantly higher percentage of events occurring during maintenance than for non-DS children (37.5% vs 15.2%; P = .048; see figure). This persistent vulnerability of children with DS to IRM throughout all treatment phases, including maintenance, was also observed in a recent large, retrospective analysis of DS-ALL patients enrolled on multiple international trials, suggesting it is not limited to a specific treatment regimen.6
The O’Connor study is significant because it establishes solid benchmark data in the largest cohort to date regarding frequency, timing, organisms, and risk factors for IRM. The study has distinct implications for DS and non-DS patients, given the significant differences in frequency and timing of IRM between the 2 groups.
Regarding management of non-DS patients, key points include the heightened risk for IRM during induction and other intensive phases of treatment, the predominance of respiratory and catheter-associated bloodstream infections, and the occurrence of death within 48 hours of initial presentation in 55% of the IRM cases. These findings highlight the critical importance of vigilance by families and providers during intensive phases of therapy, meticulous care of central venous catheters, and rapid, aggressive management of infections. As the authors point out, their study also highlights the need for further investigations to both characterize and reduce IRM in ALL. Our current portrait of IRM is limited by difficulties comparing studies because of varying definitions of key measures such as invasive fungal infections and catheter-associated bloodstream infections. It is also limited by the scope of data captured, which often lacks information about risk factors such as neutropenia, body mass index, hyperglycemia, and other comorbidities. Finally, data are lacking on how best to combat IRM. There is no current intergroup consensus on front-line treatment of ALL regarding the role of supportive measures such as IgG replacement and antibiotic and antifungal prophylaxis, or the risk-benefit ratio of treatment components such as monthly steroid pulses and an extra year of maintenance therapy for boys. Several ongoing cooperative group studies may provide answers to these questions in the future.
Regarding management of DS patients, the 12-fold increased risk for IRM drew attention during the conduct of the UKALL 2003 trial, and an amendment in 2009 implemented numerous modifications to treatment and supportive care.7 Similar unacceptably high IRM was also observed around this time on the Children’s Oncology Group (COG) standard-risk and high-risk trials, and safety amendments to these trials were also implemented.8 These modifications reduced subsequent induction mortality on UKALL 20037 and the COG standard-risk trial,8 although further events on the COG high-risk trial led to its eventual closure to patients with DS. The largely improved outcomes after these safety amendments are encouraging, but further follow-up and analysis are needed to determine whether these modifications result in an overall reduction in IRM for children with DS-ALL, and if so, whether this occurred without a corresponding increase in the risk for relapse. Because children with DS-ALL have shown an increased risk for relapse as well as IRM on several recent protocols,6,9 any treatment modifications to mitigate IRM must be carefully designed in consideration of the competing risk for disease relapse. Introduction of targeted therapies with relatively low systemic toxicity, such as Janus kinase inhibitors, based on the unique molecular features of DS-ALL, is an avenue that may eventually enable safe reductions in conventional chemotherapy.10
As survival has improved in pediatric ALL, the need for efforts to reduce IRM is clear. This work by O’Connor and colleagues is a valuable contribution to the field, demonstrating the importance of capturing well-defined toxicity data on large cooperative group trials and identifying important aspects of IRM that will guide future efforts to more effectively prevent and treat infections during the treatment of ALL.
Conflict-of-interest disclosure: The author declares no competing financial interests.