Key Points
Healthy human BM is enriched for PC lacking CD19 that express a prosurvival and distinctly mature phenotype.
CD19− PC resist mobilization into blood during immune responses after vaccination as well as B-cell depletion with rituximab.
Abstract
Specific serum antibodies mediating humoral immunity and autoimmunity are provided by mature plasma cells (PC) residing in the bone marrow (BM), yet their dynamics and composition are largely unclear. We here characterize distinct subsets of human PC differing by CD19 expression. Unlike CD19+ PC, CD19− PC were restricted to BM, expressed predominantly IgG, and they carried a prosurvival, distinctly mature phenotype, that is, HLA-DRlowKi-67−CD95lowCD28+CD56+/−, with increased BCL2 and they resisted their mobilization from the BM after systemic vaccination. Fewer mutations within immunoglobulin VH rearrangements of CD19− BMPC may indicate their differentiation in early life. Their resistance to in vivo B-cell depletion, that is, their independency from supply with new plasmablasts, is consistent with long-term stability of this PC subset in the BM. Moreover, CD19− PC were detectable in chronically inflamed tissues and secreted autoantibodies. We propose a multilayer model of PC memory in which CD19+ and CD19− PC represent dynamic and static components, respectively, permitting both adaptation and stability of humoral immune protection.
Introduction
Protective and autoreactive serum antibodies (sAb) are essential for immune protection and contribute to autoimmunity, respectively. Ab are secreted by terminally differentiated B lymphocytes, termed plasma cells (PC), and their immediate precursors, the plasmablasts (PB). Although PB generated in acute B-cell responses transiently contribute to sAb, the stability of specific sAb through extended periods may reflect long-term persistence of functional PC. Indeed, the bone marrow (BM) contains a population of continuously, perhaps indefinitely resting, “long-lived” PC, which are thought to be maintained within a hypothetical niche, providing them with survival signals such as IL-6, APRIL, and CXCL12.1-3 Possibly as a result of successful residence in a survival niche, PC express the antiapoptotic transcription factor Bcl-24,5 as a molecular trait of long-term PC survival. In humans, long-term sAb titer half-lives differ by specificity ≤100-fold,6 possibly reflecting differential survival patterns among PC subsets that maintain these titers.
We here characterize human PC lacking CD19 expression. These were strongly enriched in the BM and they expressed an antiapoptotic and distinctly mature phenotype, sharing similarities with murine long-lived PC.
On mature B cells, CD19 is a coreceptor of the B-cell receptor and regulates its signaling.7 Its expression appears as solely regulated by PAX5.8,9 During PC differentiation, PAX5 is downregulated,10 whereas CD19 is apparently lost only from a subset of PC. Lack of CD19 has been described as a hallmark of malignant PC.11 However, CD19+ and CD19−, including CD19−CD56+ PC have been reported in healthy BM,12-16 but their potential significance has remained unexplored.
Materials and methods
Detailed information is provided in the online supplement.
Blood and tissue cells
Single leukocyte or mononuclear cell suspensions were prepared from BM, tonsil, spleen, kidney, and blood according to standard protocols. Some blood samples were analyzed after vaccination against tetanus/diphtheria (TetDiph) or 2009 H1N1 influenza (Pandemrix). The ethics committees of the Charité University Medicine, University of Gothenburg and Rostock University approved the studies in accordance with the Declaration of Helsinki. Informed consent was obtained by the patients for the analyses.
Flow cytometry and cell sorting
Gene expression profiling
CD19+ and CD19− BMPC mRNA was isolated, stored, processed, and subjected to HG U133 plus 2.0 chips for global gene expression profiling (GEO accession number GSE56464).
Reagents
Rabbit antithymocyte globulin (rATG) was purchased from Fresenius Biotech, Gräfelfing, Germany, and from Genzyme, Naarden, The Netherlands, and it was labeled in house with Cy5 and PE, respectively. Rituximab (RTX) (Roche, Grenzach-Wyhlen, Germany) and bortezomib (Velcade, Millennium Pharmaceuticals Cambridge, MA) were used in cell-culture experiments. Additionally, RTX and intravenous immunoglobulin (Octapharma, Langenfeld, Germany) were labeled with fluorescein isothiocyanate.
EliSpot
EliSpot technique was used to detect functional antibody-secreting cells according to established protocols.19
Single-cell sorting and reverse transcriptase-polymerase chain reaction
Individual PB and PC were sorted into 96 well plates using a FacsAria (BD Biosciences). VHDJHC genes were amplified, sequenced, and analyzed using Chromas 2.33 Sequence Viewer (Chromas Technelysium, Helensvale, Australia), the BLAST database of the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov), and JOINSOLVER (http://joinsolver.niaid.nih.gov/) according to previously published protocols.18
Immunohistology
Cryosections of synovial biopsies were stained for CD138 and CD19. Nuclei were counterstained with DAPI. Images were acquired using a fluorescence microscope (AxioImager Z1) equipped with a charge-coupled device camera (AxioCam MRm) and processed with Axiovision software (Carl Zeiss MicroImaging, Thornwood, NY).
Statistical analysis
FlowJo (TreeStar, Ashland, OR) version 7.2.5 and GraphPad Prism 6 (GraphPad Software, San Diego, CA) were used to analyze data. Statistical significance was calculated using the Mann-Whitney U test for comparisons between patient groups at different time points. The Wilcoxon test was used to compare paired observations (both 95% confidence interval, 2-tailed). Spearman’s rank correlation analysis (2-tailed, 95% confidence interval) was performed. Levene’s test was used in SPSS v19 (IBM, Ehningen, Germany) to compare variances.
Results
The human BM is enriched for CD19− PC and represents their major storage site
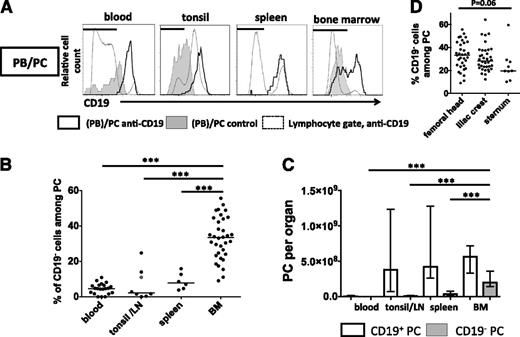
First, the expression of CD19 by PC in peripheral blood, BM, tonsil, and spleen was analyzed by flow cytometry, and frequencies as well as total numbers of CD19+ and CD19− PC residing in these tissues were determined20 (Figure 1). PC (including PB) were identified by high expression of CD38, CD138, or CD27, or combinations thereof, expressed CD20low/− and showed characteristically increased forward scatter and sideward scatter signals compared with other lymphocytes (supplemental Figure 1). Their PC identity was confirmed by the expression of cytoplasmic (cyt) immunoglobulin14,17 (Figure 2A) and for both, CD19− and CD19+ BMPC, by spontaneous secretion of antibodies as detected by Elispot (supplemental Figure 2). CD19− PC were detected as the numerically smaller PC population in the BM and at significantly lower frequencies in spleen, tonsil, and blood (Figure 1A). On average, 33% (median, range 9% to 56%) of PC in the BM did not express CD19, compared with 2% in tonsils (0% to 25%), 8% in spleens (3% to 16%), and 5% in peripheral blood (0% to 11%, Figure 1B, P < .001), respectively. Concurrently, the BM contained the majority of CD19− PC (median 2.0 × 108 cells) among the tissues analyzed, more than the blood, spleen, and tonsils altogether (0.5 × 108 cells) (Figure 1C). Frequencies of CD19− PC varied but were not significantly different between anatomic BM sites analyzed, that is, sternum (median 20%), femoral head (33%), and iliac crest (28%, Figure 1D).
The human BM is the major site of PCs lacking expression of CD19. (A) CD19 expression by PB/PC (black histograms) was analyzed by flow cytometry in peripheral blood, tonsil, lymph node (LN), BM, and spleen samples (supplemental Figure 1). CD19 expression by total lymphocytes (dashed lines) and isotype control-stained PC (shaded) are shown for comparison in representative histograms. Horizontal bars indicate gates used to determine frequencies of PC lacking CD19. Frequencies of CD19− PC among total PC were determined (B, median values are indicated from 21 blood samples, 6 spleen samples, 1 LN/7 tonsil samples, and 34 femoral head BM samples), and numbers of CD19− and CD19+ PC per organ were extrapolated for 21 blood samples, 6 spleen samples, 1 LN/7 tonsil samples, and 25 femoral head BM based on20 (C, median values and interquartile range shown). Significance levels (***P < .001) obtained from Mann-Whitney U tests are shown for comparisons between BM data and other tissues. The grey data point in (B) indicates a parotid LN. (D) Percentages of PC lacking CD19 expression were analyzed in BM samples from iliac crest (36 samples), femoral head (34 samples), and sternum (9 samples). Kruskal-Wallis testing was used for statistical analysis. Consistent frequencies of CD19− PC were determined using various anti-CD19 clones (supplemental Figure 1F).
The human BM is the major site of PCs lacking expression of CD19. (A) CD19 expression by PB/PC (black histograms) was analyzed by flow cytometry in peripheral blood, tonsil, lymph node (LN), BM, and spleen samples (supplemental Figure 1). CD19 expression by total lymphocytes (dashed lines) and isotype control-stained PC (shaded) are shown for comparison in representative histograms. Horizontal bars indicate gates used to determine frequencies of PC lacking CD19. Frequencies of CD19− PC among total PC were determined (B, median values are indicated from 21 blood samples, 6 spleen samples, 1 LN/7 tonsil samples, and 34 femoral head BM samples), and numbers of CD19− and CD19+ PC per organ were extrapolated for 21 blood samples, 6 spleen samples, 1 LN/7 tonsil samples, and 25 femoral head BM based on20 (C, median values and interquartile range shown). Significance levels (***P < .001) obtained from Mann-Whitney U tests are shown for comparisons between BM data and other tissues. The grey data point in (B) indicates a parotid LN. (D) Percentages of PC lacking CD19 expression were analyzed in BM samples from iliac crest (36 samples), femoral head (34 samples), and sternum (9 samples). Kruskal-Wallis testing was used for statistical analysis. Consistent frequencies of CD19− PC were determined using various anti-CD19 clones (supplemental Figure 1F).
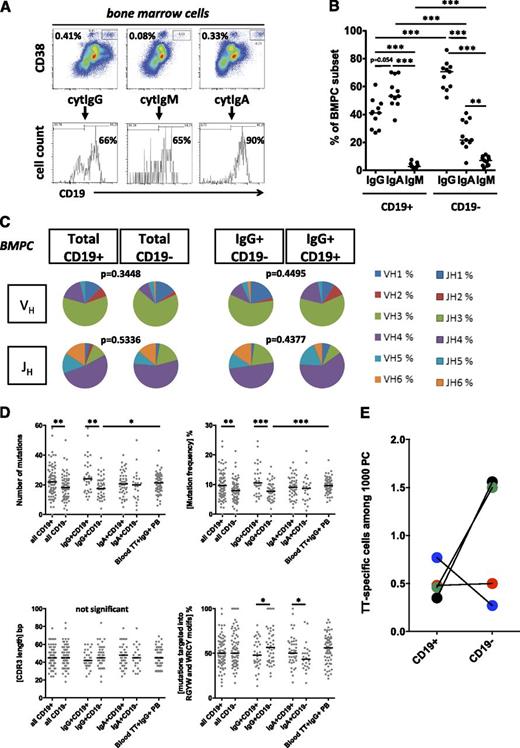
CD19− BMPC express IgG with moderately mutated VH gene rearrangements and secrete antibodies commonly found in blood serum. (A) BMPC were analyzed for expression of CD19 and cyt IgG, IgM, and IgA. Summarized results from 11 donors are shown in (B) and were compared using the Mann-Whitney U test, ***P < .001. Median values are indicated. (C-D) Individual fluorescence-activated cell sorter (FACS)-sorted CD19+ and CD19− BMPC from 3 individuals were subjected to nested single-cell reverse transcriptase-polymerase chain reaction for amplification of VHDJHC gene rearrangements.18 A total of 82 sequences from CD19+ PC and 72 sequences from CD19− BMPC were analyzed: 33 CD19+IgG+, 44 CD19−IgG+, 46 CD19+IgA+, and 24 CD19−IgA+ sequences (and 7 IgM sequences, not separately shown). Sequences were analyzed for immunoglobulin isotype (supplemental Figure 2B) for VH and JH family use. Distributions were compared by χ2 test; respective P values are indicated (C), for absolute numbers and frequencies of somatic mutations per VH sequence, and for CDR3 length and mutations located within RGYW/WRCY hotspot motifs (D). Data from 65 tetanus toxoid (TT)-specific PB18 from blood 1 week after intramuscular TetDiph vaccination from 3 donors, respectively, are shown for comparison. After χ2 tests did not show significant differences in VHJHC segment use and CDR3 length distribution among different individuals’ samples, data were pooled and displayed together. Mann-Whitney U test was used to analyze data in (D) (*P < .05; **P < .01; ***P < .001). Median values are indicated. (E) Frequencies of antibody-secreting PC specific for TT were determined by Elispot among 4 pairs of FACS-sorted CD19+ and CD19− BMPC (donors were aged 37, 52, 62, and 72 years). Median values are indicated.
CD19− BMPC express IgG with moderately mutated VH gene rearrangements and secrete antibodies commonly found in blood serum. (A) BMPC were analyzed for expression of CD19 and cyt IgG, IgM, and IgA. Summarized results from 11 donors are shown in (B) and were compared using the Mann-Whitney U test, ***P < .001. Median values are indicated. (C-D) Individual fluorescence-activated cell sorter (FACS)-sorted CD19+ and CD19− BMPC from 3 individuals were subjected to nested single-cell reverse transcriptase-polymerase chain reaction for amplification of VHDJHC gene rearrangements.18 A total of 82 sequences from CD19+ PC and 72 sequences from CD19− BMPC were analyzed: 33 CD19+IgG+, 44 CD19−IgG+, 46 CD19+IgA+, and 24 CD19−IgA+ sequences (and 7 IgM sequences, not separately shown). Sequences were analyzed for immunoglobulin isotype (supplemental Figure 2B) for VH and JH family use. Distributions were compared by χ2 test; respective P values are indicated (C), for absolute numbers and frequencies of somatic mutations per VH sequence, and for CDR3 length and mutations located within RGYW/WRCY hotspot motifs (D). Data from 65 tetanus toxoid (TT)-specific PB18 from blood 1 week after intramuscular TetDiph vaccination from 3 donors, respectively, are shown for comparison. After χ2 tests did not show significant differences in VHJHC segment use and CDR3 length distribution among different individuals’ samples, data were pooled and displayed together. Mann-Whitney U test was used to analyze data in (D) (*P < .05; **P < .01; ***P < .001). Median values are indicated. (E) Frequencies of antibody-secreting PC specific for TT were determined by Elispot among 4 pairs of FACS-sorted CD19+ and CD19− BMPC (donors were aged 37, 52, 62, and 72 years). Median values are indicated.
CD19− BMPC are enriched for IgG expression and carry fewer mutations in their VHDJH gene rearrangements compared with CD19+ BMPC
BMPC have been suggested as the major source of IgG in rodents.21,22 We therefore analyzed the expression of the antibody isotype produced by individual human BMPC so as to estimate the contribution of CD19+ vs CD19− BMPC to systemic IgG production (Figure 2A). In 11 BM samples analyzed, 71% of CD19− PC expressed IgG (median, range 52% to 86%), followed by IgA (22%, 5% to 41%) and IgM (7%, 2% to 11%). Within CD19+ BMPC, IgA was most abundantly expressed (53% of PC, 36% to 70%), followed by IgG (41%, 27% to 61%) and IgM (3%, 1% to 8%) (Figure 2B). Isotype-specific Elispot and analyses of IgVHDJHC gene rearrangements amplified from individual BMPC confirmed these results (supplemental Figure 2A-B). To gain insight into the composition of BMPC subsets and the differentiation of individual CD19+ and CD19− BMPC, the same IgVHDJHC sequences were used to assess the use of VH and JH gene family members, the acquisition of somatic mutations within IgVH gene rearrangements, and CDR3 region lengths. CD19+ and CD19− BMPC used VH and JH gene family members at comparable frequencies, irrespective of whether total or IgG+ BMPC were analyzed (Figure 2C), indicating similar selection of the B cells that differentiated into CD19+ and CD19− BMPC, respectively. A total of 95% of the sequences analyzed represented unique VHDJH gene rearrangements, reflecting a polyclonal VH and JH gene repertoire. Clonally related BMPC were uncommon. A total of 2 clonally related sequence pairs were detected in 1 of 3 donors, with 1 clone shared between CD19− and CD19+ BMPC (supplemental Table I).
Although mutation numbers and frequencies did not differ between CD19+ and CD19− BMPC of the IgA isotype, CD19−IgG+ PC showed markedly lower total mutations (median 18 vs 24 mutations per VH sequence, P = .0022) and mutation frequencies (7.7% vs 10.5%, P = .0007) but not significantly longer CDR3 sequences (median 45 base pair vs 42 base pair, P = .1583) compared with CD19+IgG+ BMPC (Figure 2D). TT-specific IgG+ PB circulating 1 week after systemic vaccination are thought to migrate to the BM and represent potential precursors of BMPC.23 Indeed, TT-specific cells were found among both BMPC subsets in all 4 donors tested at frequencies between 0.27‰ and 1.56‰ (Figure 2E). However, only CD19+IgG+ BMPC showed similar mutation frequencies compared with blood TT-specific PB (9.7% vs 10.5%, P = .1563), whereas IgG+CD19− BMPC showed a significantly lower mutation frequency (7.7%, P = .0005; Mann-Whitney U test, Figure 2D). This is consistent with our hypothesis that in adults, TT-specific PB of the blood could represent precursors of CD19+ BMPC, but not precursors of CD19− BMPC.
CD19− BMPC express a prosurvival and uniquely advanced differentiated PC phenotype
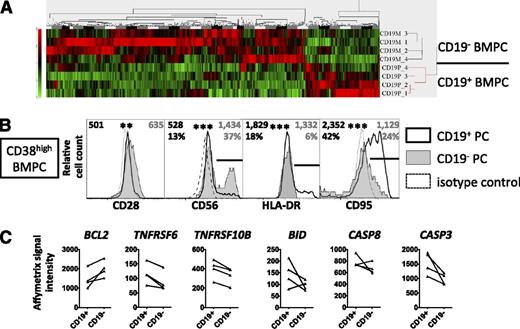
Global gene expression profiles of FACS-sorted CD19+ and CD19− PC (supplemental Figure 4) showed significantly different signals in 859 Affymetrix probe sets corresponding to 689 differentially expressed genes (Figure 3A; supplemental Table IIIA). Among these, signal intensities for molecules otherwise unrelated to B cells, CD28 and CD56, were significantly upregulated in CD19− BMPC, which was confirmed by FACS analyses. Vice versa, HLA-DR, previously described as a marker of PB17,23 and the death receptor CD95,24,25 were downregulated in CD19− BMPC (both P < .001) (Figure 3B). The low expression of CD95 by CD19− BMPC was accompanied by lower Affymetrix signal intensities of proapoptotic mediators such as TNFRSF10B26 and BID, the apoptosis effector caspases 3 and 8, whereas higher signals were found for BCL2L11 and the antiapoptotic factor BCL227 in CD19− BMPC compared with CD19+ BMPC (Figure 3C; supplemental Table IIIB). Receptors for the PC survival factors IL-6, CXCL12, BAFF, and APRIL, as well as a critical mediator of murine PC survival, Mcl-1,28 were expressed at comparable levels in human CD19+ and CD19− BMPC (supplemental Table IIIB-C). To test whether the differences found in apoptosis regulators translate into a survival advantage for CD19− or CD19+ BMPC, we analyzed the survival of FACS-sorted CD19+ and CD19− BMPC in vitro. Most PC died within 24 hours after sorting, yet consistently higher frequencies were recovered for viable CD19− compared with CD19+ BMPC after 3 to 9 days of culture. For example, 11.1% vs 6.1%, 5.7% vs 2.4%, and 2.7% vs 1.8%, respectively, were recovered of cultured CD19− vs CD19+ BMPC after 8 to 9 days (3 experiments). Thus the vast majority of CD19− BMPC expressed the phenotype of uniquely differentiated PC, that is, CD38highCD138+CD19−CD28+HLA-DRlowCD56+/−CD95low with increased BCL2 and enhanced survival in vitro, suggesting their enhanced survival capacity in vivo and their involvement in long-term sAb maintenance.
The phenotype of CD19− BMPC indicates terminal PC differentiation and prosurvival capacity. (A) CD19+ and CD19− BMPC were isolated from 4 donors using a combined magnetic-activated cell-sorting/FACS protocol (supplemental Figure 4) and were subjected to global gene expression profiling. A heat map resulting from unsupervised clustering of data of 859 significantly differentially expressed probe sets reflecting 689 genes (supplemental Table IIIA) according to high-performance chip data analysis (HPCDA) after Bonferroni correction is shown. (B) CD19+ and CD19− BMPC (CD38highCD3−CD14−DAPI−) were analyzed by flow cytometry for the expression of CD28, CD56, HLA-DR, or CD95. Histograms representative of 8 (CD28), 14 (CD56), 18 (HLA-DR), or 19 (CD95) donors are shown. Numbers represent median values of the geometric mean fluorescence intensity values of all donors analyzed, frequencies represent median values of frequencies of PC expressing a marker gated as illustrated by the black bars in the histogram plots, where applicable (gray, CD19− BMPC; black CD19+ BMPC). Both median fluorescence intensity and frequency data were compared using the Wilcoxon test (**P < .01; ***P < .001). (C) Affymetrix signal intensity data for selected molecules involved in apoptosis regulation not contained in the list of differentially expressed genes as delivered by HPCDA, that is, BCL2 (B-cell lymphoma 2, probeset 203685_at), TNFRSF6 (CD95), TNFRSF10B (tumor necrosis factor-related apoptosis-inducing ligand receptor 2), BID (BH3 interacting-domain death agonist), and for caspases 8 and 3 (supplemental Table IIIB), were extracted from global gene expression analysis of CD19+ and CD19− BMPC. A distinct probeset for BCL2 (203685_at) was identified as differentially expressed by HPCDA. Student t test P values (BCL2, 0.05; TNFRSF6, 0.01; TNFRSF10B, 0.10; BID, 0.002; CASP8, 5.3 × 10−5; and CASP3, 1.1 × 10−5, were not considered significant according to the significance level of 0.05 corrected for multiple comparisons in the overall analysis (P = 2.29 × 10−8).
The phenotype of CD19− BMPC indicates terminal PC differentiation and prosurvival capacity. (A) CD19+ and CD19− BMPC were isolated from 4 donors using a combined magnetic-activated cell-sorting/FACS protocol (supplemental Figure 4) and were subjected to global gene expression profiling. A heat map resulting from unsupervised clustering of data of 859 significantly differentially expressed probe sets reflecting 689 genes (supplemental Table IIIA) according to high-performance chip data analysis (HPCDA) after Bonferroni correction is shown. (B) CD19+ and CD19− BMPC (CD38highCD3−CD14−DAPI−) were analyzed by flow cytometry for the expression of CD28, CD56, HLA-DR, or CD95. Histograms representative of 8 (CD28), 14 (CD56), 18 (HLA-DR), or 19 (CD95) donors are shown. Numbers represent median values of the geometric mean fluorescence intensity values of all donors analyzed, frequencies represent median values of frequencies of PC expressing a marker gated as illustrated by the black bars in the histogram plots, where applicable (gray, CD19− BMPC; black CD19+ BMPC). Both median fluorescence intensity and frequency data were compared using the Wilcoxon test (**P < .01; ***P < .001). (C) Affymetrix signal intensity data for selected molecules involved in apoptosis regulation not contained in the list of differentially expressed genes as delivered by HPCDA, that is, BCL2 (B-cell lymphoma 2, probeset 203685_at), TNFRSF6 (CD95), TNFRSF10B (tumor necrosis factor-related apoptosis-inducing ligand receptor 2), BID (BH3 interacting-domain death agonist), and for caspases 8 and 3 (supplemental Table IIIB), were extracted from global gene expression analysis of CD19+ and CD19− BMPC. A distinct probeset for BCL2 (203685_at) was identified as differentially expressed by HPCDA. Student t test P values (BCL2, 0.05; TNFRSF6, 0.01; TNFRSF10B, 0.10; BID, 0.002; CASP8, 5.3 × 10−5; and CASP3, 1.1 × 10−5, were not considered significant according to the significance level of 0.05 corrected for multiple comparisons in the overall analysis (P = 2.29 × 10−8).
CD19+ and CD19− BMPC exhibit different stability in vivo
Next we studied the proliferation and survival of CD19+ and CD19− BMPC by analyzing Ki-67 expression and the impact of in vivo B-cell depletion by anti-CD20 treatment leading to suppression of systemic PB generation.
Different from peripheral blood PB/PC, Ki-67+ cells were infrequent among BMPC, that is, more than 95% of PC in the BM represented resting cells. However, CD19+ BMPC contained significantly more Ki-67+ cells compared with CD19− BMPC. Frequencies of Ki-67+ cells among CD19− BMPC were 0.9% (median, compared with 0.0% control stained cells), and 3.6% within CD19+ BMPC (compared with 0.5%, respectively, P < .05) (Figure 4A).
CD19+ and CD19− BMPC subsets exhibit different ex vivo proliferation and in vivo persistence. (A) CD19+ and CD19− BMPC and peripheral blood PB/PC were analyzed for the expression of Ki-67 after cell fixation with formaldehyde and permeabilization with saponin. Frequencies of Ki-67+ cells are shown and were compared between blood PB/PC (6 samples, steady state) and BMPC subsets (Mann-Whitney U test; ***P < .001) and among pairs of BM CD19+ and CD19− PC (Wilcoxon test; *P < .05). Filled circles, Ki-67 staining (10 samples); open circles, control staining (available for 7 samples). Both BMPC subsets show frequencies of Ki-67+ cells significantly exceeding frequencies of isotype control stained cells (Mann-Whitney U test; both P < .01). Median values are indicated. (B) BM samples of 23 patients with rheumatoid arthritis treated with 2 × 1 g RTX (a chimeric anti-CD20 antibody) were analyzed for the presence of CD138+CD19+ and CD138+CD19− PC twice, once before, and once again 1 to 3 months after treatment. PC were detected by flow cytometry, and their numbers were extrapolated according to Trepel20 and were analyzed using the Wilcoxon test; *P < .05. Lines between data points indicate the trend shown by individual patients. Mean numbers of 3.10 × 108 (standard deviation [SD], 1.76 × 108) CD19+ and 1.36 × 108 (SD, 0.85 × 108) CD19− BMPC compare with 2.24 × 108 (SD, 1.69 × 108) CD19+ and 1.06 × 108 (SD, 0.93 × 108) CD19− BMPC after therapy, respectively.
CD19+ and CD19− BMPC subsets exhibit different ex vivo proliferation and in vivo persistence. (A) CD19+ and CD19− BMPC and peripheral blood PB/PC were analyzed for the expression of Ki-67 after cell fixation with formaldehyde and permeabilization with saponin. Frequencies of Ki-67+ cells are shown and were compared between blood PB/PC (6 samples, steady state) and BMPC subsets (Mann-Whitney U test; ***P < .001) and among pairs of BM CD19+ and CD19− PC (Wilcoxon test; *P < .05). Filled circles, Ki-67 staining (10 samples); open circles, control staining (available for 7 samples). Both BMPC subsets show frequencies of Ki-67+ cells significantly exceeding frequencies of isotype control stained cells (Mann-Whitney U test; both P < .01). Median values are indicated. (B) BM samples of 23 patients with rheumatoid arthritis treated with 2 × 1 g RTX (a chimeric anti-CD20 antibody) were analyzed for the presence of CD138+CD19+ and CD138+CD19− PC twice, once before, and once again 1 to 3 months after treatment. PC were detected by flow cytometry, and their numbers were extrapolated according to Trepel20 and were analyzed using the Wilcoxon test; *P < .05. Lines between data points indicate the trend shown by individual patients. Mean numbers of 3.10 × 108 (standard deviation [SD], 1.76 × 108) CD19+ and 1.36 × 108 (SD, 0.85 × 108) CD19− BMPC compare with 2.24 × 108 (SD, 1.69 × 108) CD19+ and 1.06 × 108 (SD, 0.93 × 108) CD19− BMPC after therapy, respectively.
Treatment with RTX resulting in CD20+ B-cell depletion resulted in a different impact on CD19+ vs CD19− BMPC in vivo. After such treatment, CD19+ B cells were absent from peripheral blood,29 and numbers of CD19+ but not CD19− BMPC were significantly reduced (23 patients) when CD19+ and CD19− BMPC were analyzed simultaneously from BM samples taken before and 1 to 3 months after therapy, respectively. Although we noted some heterogeneity of BMPC subsets across individual donors, the overall data indicate that CD19− BMPC appear to be more resistant to peripheral B-cell depletion by RTX, that is, they do not depend, or they depend less, on the supply of new PB (Figure 4B). Thus CD19+ and CD19− BMPC showed different cell proliferation and population persistence characteristics in independent in vivo and ex vivo analyses, identifying CD19+ BMPC as the more dynamic and CD19− BMPC the rather static PC population, respectively. Although these data capture the dynamics of BMPC subsets during steady state, it remained unclear whether or not CD19− BMPC are induced during or mobilized into blood after systemic immune responses.
CD19+, but not CD19− (HLA-DRlow) PC appear in the blood after vaccination
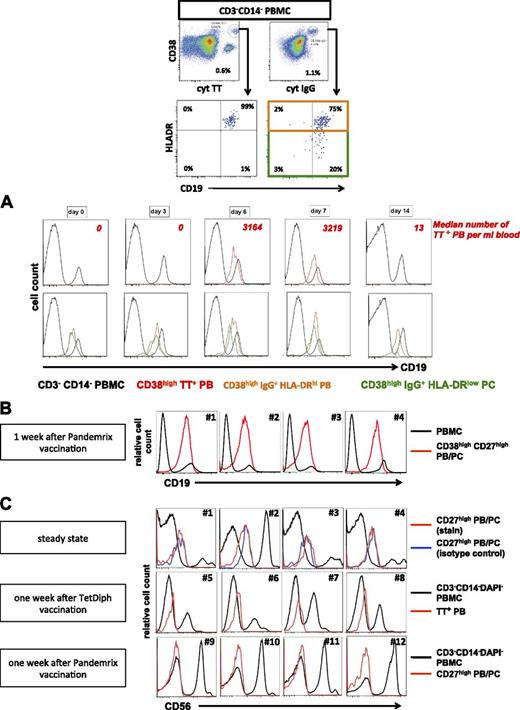
One week after TetDiph vaccination, mature HLA-DRlow PC emerge in human blood simultaneously with proliferating and migratory, antigen-specific PB.5,17,23 We subsequently analyzed CD19 and CD56 expression by these cells to identify whether circulating CD19−CD56+/−HLA-DRhigh PB were generated in such an immune response and/or whether mature CD19−CD56+/−HLA-DRlow PC were mobilized into the blood after vaccination.23 In accordance with previous studies,17,23 intramuscular TetDiph vaccination induced an increase of IgG+HLA-DRhigh PB in peripheral blood 1 week after vaccination, including TT-specific PB. Throughout the course of the immune response, few if any TT-specific PB expressed the CD19-negative phenotype, as did total HLA-DRhigh expressing PB and HLA-DRlow PC (Figure 5A). Similar results were obtained after vaccination against H1N1 with Pandemrix, which led to a substantial induction of peripheral blood PB/PC 1 week after vaccination (30 290 to 95 480 PB/PC/mL, 4 donors) (Figure 5B).
CD19+ but not CD19− PB and PC are detectable in peripheral blood after vaccination. (A) TT-specific PB, IgG+HLA-DRhigh PB and IgG+HLA-DRlow PC were detected in peripheral blood by flow cytometry as shown (1 week after vaccination) and were analyzed for expression of CD19 in individuals before and at indicated time points after intramuscular vaccination against TetDiph. The analysis shown is representative of 9 individuals immunized with TetDiph vaccine. Numbers of TT-specific PB/mL blood are indicated to illustrate kinetics of the TT-specific PB response. (B) CD19 expression by PB/PC from 4 individuals 1 week after vaccination against 2009 H1N1 influenza with Pandemrix. (C) CD56+ PB and PC are absent from peripheral blood before and after vaccination. Representative analyses are shown for circulating PB/PC during steady state (#1 to #4), 1 week after TetDiph vaccination (#5 to #8) and 1 week after Pandemrix vaccination (#9 to #12).
CD19+ but not CD19− PB and PC are detectable in peripheral blood after vaccination. (A) TT-specific PB, IgG+HLA-DRhigh PB and IgG+HLA-DRlow PC were detected in peripheral blood by flow cytometry as shown (1 week after vaccination) and were analyzed for expression of CD19 in individuals before and at indicated time points after intramuscular vaccination against TetDiph. The analysis shown is representative of 9 individuals immunized with TetDiph vaccine. Numbers of TT-specific PB/mL blood are indicated to illustrate kinetics of the TT-specific PB response. (B) CD19 expression by PB/PC from 4 individuals 1 week after vaccination against 2009 H1N1 influenza with Pandemrix. (C) CD56+ PB and PC are absent from peripheral blood before and after vaccination. Representative analyses are shown for circulating PB/PC during steady state (#1 to #4), 1 week after TetDiph vaccination (#5 to #8) and 1 week after Pandemrix vaccination (#9 to #12).
Notably, CD56+ PB or PC were not detectable in the blood during steady state (11 donors) and were also absent from the blood 1 week after vaccination with TetDiph or Pandemrix vaccine (5 and 4 donors, respectively) (Figure 5C), confirming the absence from blood of CD56+ PC that are routinely detectable in BM, especially within the CD19− BMPC subset.
Taken together, CD19− PC including CD56+ PC were restricted to BM residence also during the adaptation of the BMPC pool after vaccination in adulthood. Thus downregulation of CD19 and upregulation of CD56 by PC apparently occurs in situ after their immigration into the BM, suggesting ongoing differentiation of PC within the BM.
CD19− PC accumulate in inflamed tissue and are affected by rATG and bortezomib treatment
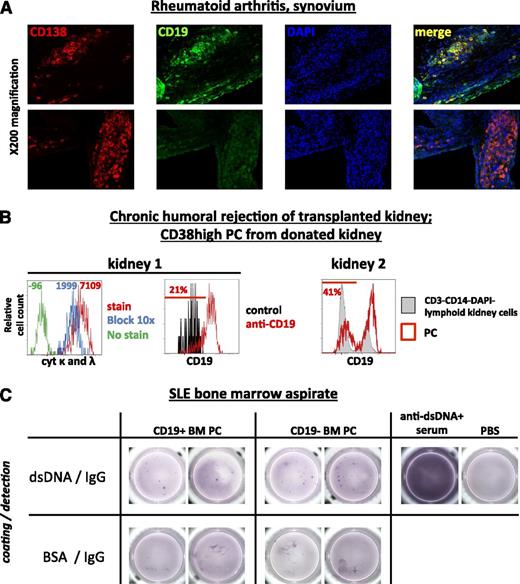
Whereas CD19− PC were apparently restricted to the BM in healthy individuals, CD19− PC were also detectable in inflamed tissues, that is, synovial biopsies from patients with rheumatoid arthritis (2 samples) as well as in kidneys explanted after chronic humoral graft rejection (Figure 6A-B). In a BM sample from one systemic lupus erythematosus (SLE) patient, dsDNA-specific IgG-secreting cells were detectable among both, CD19+ and CD19− BMPC (Figure 6C), at frequencies of 0.8 and 1.3 PC per 10 000 total CD19+ and CD19− BMPC, respectively.
CD19− PC are detectable at sites of chronic inflammation and can secrete autoantibodies. (A) Synovial biopsies from 2 patients with rheumatoid arthritis were analyzed by immunofluorescence histology for the presence of CD138+DAPI+CD19+ and CD138+DAPI+CD19− PC (1 out of 2 cases is shown). Upper and lower panels originate from the identical staining and section. (B) Kidney tissue from 2 patients after humoral rejection was digested by collagenase V, and mononuclear cells were analyzed by flow cytometry for the presence of CD19+ and CD19− PC. PC were identified using high expression of CD38 within a wide lymphocyte gate and electronic depletion of CD3+ cells, CD14+ cells, and dead cells. In 1 of the 2 cases, CD19 expression by PC was controlled using an isotype-matched control antibody, and the PC identity was confirmed by the detection of intracellular immunoglobulin (cyt κ and λ Ab light chains). Numbers denote mean fluorescence intensities. (C) CD19+ and CD19− BMPC from 1 SLE patient (serum anti-dsDNA antibodies, 84 U/mL) were isolated and subjected to an Elispot assay detecting dsDNA-specific IgG-secreting cells among 24 000 CD19+ BMPC/well and 27 000 CD19− BMPC/well. Representative wells (from 14 dsDNA-coated replicate wells and 7 bovine serum albumin-coated replicate wells per PC subset to determine assay background) and assay controls are shown. After background subtraction, frequencies of dsDNA-specific PC were 0.8 and 1.3 PC per 10 000 total CD19+ and CD19− BMPC, respectively.
CD19− PC are detectable at sites of chronic inflammation and can secrete autoantibodies. (A) Synovial biopsies from 2 patients with rheumatoid arthritis were analyzed by immunofluorescence histology for the presence of CD138+DAPI+CD19+ and CD138+DAPI+CD19− PC (1 out of 2 cases is shown). Upper and lower panels originate from the identical staining and section. (B) Kidney tissue from 2 patients after humoral rejection was digested by collagenase V, and mononuclear cells were analyzed by flow cytometry for the presence of CD19+ and CD19− PC. PC were identified using high expression of CD38 within a wide lymphocyte gate and electronic depletion of CD3+ cells, CD14+ cells, and dead cells. In 1 of the 2 cases, CD19 expression by PC was controlled using an isotype-matched control antibody, and the PC identity was confirmed by the detection of intracellular immunoglobulin (cyt κ and λ Ab light chains). Numbers denote mean fluorescence intensities. (C) CD19+ and CD19− BMPC from 1 SLE patient (serum anti-dsDNA antibodies, 84 U/mL) were isolated and subjected to an Elispot assay detecting dsDNA-specific IgG-secreting cells among 24 000 CD19+ BMPC/well and 27 000 CD19− BMPC/well. Representative wells (from 14 dsDNA-coated replicate wells and 7 bovine serum albumin-coated replicate wells per PC subset to determine assay background) and assay controls are shown. After background subtraction, frequencies of dsDNA-specific PC were 0.8 and 1.3 PC per 10 000 total CD19+ and CD19− BMPC, respectively.
Moreover, we studied whether CD19+ and CD19− PC were affected in vitro and in vivo by therapeutics considered to target PC.30,31 CD19+ and CD19− BMPC were similarly bound by fluorescently labeled rATG whereas RTX that was used as a control bound to <10% of CD19+ BMPC but not to CD19− BMPC (supplemental Figure 3A). In vitro, the addition of the proteasome inhibitor bortezomib, but not RTX, resulted in reductions of CD19+ BMPC by 76% and of CD19− PC by 64%, respectively, within 3 days from total BM cell cultures (mean values, 3 experiments) when compared with control cultures (supplemental Figure 3B). The BM from 2 individual patients with antibody-mediated diseases treated with either bortezomib or rATG contained very low frequencies of total PC, which comprised both CD19+ and CD19− BMPC (supplemental Figure 3C-D). Thus both drugs appear principally capable to impact on BMPC (or their supportive environment) in vivo. However, the effect of both drugs on BMPC needs to be addressed more systematically to verify this finding.
These results demonstrate the presence of otherwise BM-restricted CD19− PC at sites of inflammation in antibody-mediated diseases and they provide direct evidence for their numerical reduction after treatment with rATG and bortezomib but not RTX in humans.
Discussion
Specific IgG Ab are an important line of human immune defense. The dynamics especially of the human PC pool is still minimally understood, whereas medical interventions to improve immune protection and to treat antibody-mediated diseases demand better insight into PC homeostasis.
Following their generation, differentiating PB migrate to and may become resident in the BM2,23,32 and in secondary lymphoid tissues. PC of the BM generate most sAb.21,22 Their survival depends on factors provided by cells and matrix components constituting a putative survival niche.3,5,33 A fraction of nondividing antigen-specific IgG+ PC in the BM has been described as particularly long lived.2 These are strongly implicated in the long-term maintenance of specific sAb levels (humoral memory) that is reflected in humans by the observed stability of specific sAb titers,6 including sAb specific for a long-term eradicated pathogen, smallpox.34 Long-lived PC have been coined memory PC,5 and they were shown to contribute to autoreactive sAb production, to resist cytostatic treatment, and to be maintained in inflamed kidneys in a mouse model of SLE.35,36 Human CD19− BMPC described in this study reside in the BM, and, in immunopathology, within inflamed tissue, and they show little if any signs of proliferation, thus sharing key features of mouse long-lived PC.37 Like murine long-lived Blimp-1high BMPC, notably showing reduced CD19 and HLA-DR expression,37 human BMPC appear to acquire their characteristic CD19− phenotype in situ. The nature of this developmental process remains to be delineated. In support of a survival advantage of human CD19− BMPC, they showed mostly antiapoptotic characteristics when isolated ex vivo and enhanced spontaneous survival in vitro.
CD19− BMPC further express indicators of advanced PC maturity such as CD2838,39 and CD56. In mice, CD28 is expressed by PC after downregulation of PAX5,39 and CD28 contributes to the regulation of the mature BMPC40,41 although its exact role needs to be delineated.42 Importantly, neoplastic CD28+ PC are able to modulate their own microenvironment,43 and normal PC can induce production of the PC survival factor IL-6 by stromal cells,44 that is, cells to which PC stably attach in the BM.45 The ability of a subset of PC to shape their own niche could contribute to their stability even when the niche is transiently disrupted. Indeed, some PC survive the depletion of PC-supporting basophils,46 eosinophils,47 and megakaryocytes.48 Moreover, CD56 may have a role in the stable positioning of CD56+ PC in the BM, via homotypic interaction with CD56+ osteoblasts49,50 and/or possibly mesenchymal stem cells.51,52 The association of higher CD56 expression by PC tumors with lower PC numbers in the blood and less BM infiltration or osteolysis53,54 supports that CD56 is indeed involved in the in situ arrest of PC.
CD19− BMPC also downregulated HLA-DR and appear to be protected from mobilization during immune responses, potentially contributing to the robustness and stability of this PC subset in the BM. In contrast, CD19+ BMPC could represent a source of HLA-DRlow PC that appear in blood at day 7 after systemic vaccination.23 As vaccine-induced antigen-specific PB and CD19+ BMPC share phenotypical and molecular VH sequence properties, CD19+ BMPC candidate as the primary PC subset to accommodate new PB in the adult BM.
In contrast to CD19− BMPC, CD19+ BMPC expressed a less mature phenotype (CD28-HLA-DRhigh/lowCD95+) consistent with susceptibility to apoptosis, and they contained somewhat more proliferating cells during steady-state and showed partial dependency on the presence of peripheral B cells, suggesting a more dynamic nature and possibly adaptation capacity of the CD19+ BMPC subset. Whether such BMPC proliferation plays a role for the maintenance of serum IgG in addition to regulation of immunoglobulin secretion by PC55 and PC survival remains to be addressed. Despite considerable heterogeneity in their response to B-cell depletion, CD19− BMPC did not significantly depend on the presence of peripheral B cells, thus comparing to PC surviving B-cell depletion in mice,56 in inflamed synovia57 and PC-producing protective and certain autoreactive sAb.58,59 CD20 expression by both BMPC subsets was similarly weak or absent as measured by a commercial antibody (supplemental Figure 1E). However, we identified weak binding of RTX to CD19+ BMPC, but RTX did not deplete those in vitro (supplemental Figure 3B), so that differential effects of the in vivo RTX treatment appear as unrelated to potentially residual CD20 expression by PC. Numbers of CD19− BMPC showed lower variance across donors than CD19+ BMPC, (36 samples, Levene’s test, P = .005), possibly reflecting that their numbers are a conserved biological characteristic. In conclusion, CD19− BMPC appear as a more durable memory PC differentiation stage distinct from CD19+ PC. Our data are consistent with a model in which CD19− BMPC represent a rather static population of presumably long-lived PC with a molecular survival imprint, whereas CD19+ BMPC comprise a more dynamic subset whose survival may depend on factors that do not induce increased levels of BCL2. As Bcl-2 alone is not able to prevent PC from dying,4 it may rather represent an indicator of the long-term residence of CD19− BMPC in a PC-supporting BM habitat.
Thus we propose that humoral immunity relies on distinct subsets of PC with complementary functions and different dynamics. This model can explain the robustness of sAb titers, including adaptation of the memory PC pool after immune responses. Moreover, the differing half lives of specific serum IgG titers6 on the background of limited numbers of total PC in the BM might be related to distribution of antigen-specific PC into CD19+ and CD19− PC pools, respectively.
The in situ differentiation of CD19− BMPC suggests a sequential differentiation process in which circulating PB immigrate the BM and fuel the CD19+ BMPC population, and some CD19+ BMPC may further mature into CD19− BMPC. Although the determinants of this putative differentiation pathway remain unknown, the enrichment of IgG expression and the lower mutation frequency of VH gene rearrangements of CD19− BMPC compared with CD19+ BMPC imply that such differentiation does not occur in adults regularly, because that would reflect the selection of PC with lower somatic mutations into the CD19− PC pool, which appears unlikely. A different scenario involves differentiation of CD19+ into CD19− PBMC during childhood or early life, and thereafter, once the pool of CD19− BMPC is established, the differentiation of CD19+ into new CD19− PC is either inhibited or CD19− BMPC formed later in life do not successfully establish and they die. The lower mutation frequency observed in CD19− BMPC may reflect their differentiation from (memory) B cells that experienced limited antigenic restimulation, such as in early life.60,61 CD19− BMPC observed here may reflect descendants of, for example, secondary or tertiary immune responses that persisted since their generation until the time point of sampling, because blood PB induced in primary immune responses showed apparently less somatic hypermutation62 compared with CD19−IgG+ BMPC, whereas those resulting from TetDiph booster vaccination in adults carried more somatic hypermutations. In contrast, CD19+ BMPC in the same samples might stem from more recent recall responses, and may have differentiated from memory B cells that underwent more cycles of affinity maturation, therefore showing a higher degree of somatic hypermutation. In that regard, according to national vaccination guidelines (www.rki.de), TetDiph vaccinations in adulthood (18 to 60 years) represent the seventh to 11th intentional immunization with the same antigen.
This overall scenario complies with the selection of PC for higher antigen affinity and higher mutational frequencies within IgVH gene rearrangements compared with their memory B-cell or germinal-center counterparts.63,64 Interestingly, BM samples from infants aged 5 to 7 months uniformly lacked CD19− BMPC but contained CD19+ BMPC (supplemental Figure 2C). This indicates that CD19+ PC are the first to appear in BM, and could be subject to sequential differentiation and selection of CD19+ into CD19− BMPC during childhood or adolescence.
Whereas the lower mutational frequency of CD19− BMPC could also indicate limitations in T-cell help, the presence of PC specific for a T-dependent antigen (tetanus) as well as frequent targeting of mutations into mutational hot spots argues against the view that CD19− BMPC were formed during T-cell independent responses.
Secretion of autoreactive IgG from CD19− (but also CD19+) BMPC and their detection in inflamed tissues implicate a participation of CD19− PC in the immunopathology of antibody-mediated diseases. In this regard, we provide initial data from single patients indicating that both CD19− and CD19+ BMPC were numerically reduced after treatment with the proteasome inhibitor bortezomib as well as rATG used in immunoablation protocols. Direct targeting of PC by rATG and bortezomib appears likely, because bortezomib is effective against PC tumors by inducing apoptosis, and rATG directly binds to BMPC.30,31,65,66 In vivo, these treatments can additionally impact on PC numbers indirectly by affecting their environment. As our data represent single cases, the results require validation in larger cohorts. Anti-CD19 therapies in development for treatment of patients with lymphoma and also autoimmunity67,68 are unlikely to target CD19− PC directly.
In multiple myeloma patients, many PC tumors share the phenotype we describe for normal CD19− BMPC, including expression of CD56,69 and CD28,16 and enhanced CD200 signals (supplemental Table IIID).70,71 However, CD117 is rated absent in the transcriptional profiles of CD19+ and CD19− BMPC; CD19− BMPC were polyclonal and detectable in every BM sample analyzed in this study, confirming that they were not malignant PC.
The current study improves our understanding of regulation of humoral memory at the level of PC and their subsets, and it has implications for the development of vaccines and treatment of systemic autoimmunity.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Toralf Kaiser, Katharina Raba, and Jenny Kirsch for assistance with cell sorting, and Karin Reiter, Simone Spieckermann, and the German Rheumatism Research Center Berlin Central Laboratory for excellent technical assistance. The authors thank Karin Schwarz, Kathi Thiele, and the BCRT Cell Harvesting Laboratory for the provision of samples, and the authors thank Heidi Schliemann and Andreas Grützkau from the Regine-von-Ramin-Laboratory for Molecular Rheumatology, who were responsible for processing global gene expression arrays.
This work was supported by German Research Foundation grants Do 491/8-1/2 (SPP Immunobone), Do491/7-3, SFB633/TP A14, SFB650/TP12, Mu844/13-1, the DGRh start-up grant, and by the Berlin Senate through Charité University Medicine.
Authorship
Contribution: H.E.M., I.W., D.F., C.G., M.B., J.R.G., T.A., S.S., K.L., A.A.K., R.E., and T.S. performed experiments and analyzed results; H.E.M., I.W., and A.A.K. created the figures; H.E.M. and T.D. planned research and experimental strategy; H.E.M. wrote the manuscript; T.D., A.R., I.W., K.L., C.G., D.F., and J.R.G. evaluated the data, edited the manuscript, and provided advice; and T.A., R.E., T.S., M.D., M.B., and C.P. provided vital material or data for the study and discussed results.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for H.E.M. is the Human Immune Monitoring Center, Institute for Immunity, Transplantation and Infection, Stanford School of Medicine, Stanford University, Stanford, CA.
The current affiliation for K.L. is Mucosal Immunology, Department of Experimental Medical Science, Lund University, Lund, Sweden.
Correspondence: Henrik Mei, The Human Immune Monitoring Center (HIMC), Institute for Immunity, Transplantation and Infection, Stanford University School of Medicine, 299 Campus Dr, Fairchild Science Building, Room D033, Stanford, CA 94305; e-mail: hemei@stanford.edu or mei@drfz.de.

![Figure 4. CD19+ and CD19− BMPC subsets exhibit different ex vivo proliferation and in vivo persistence. (A) CD19+ and CD19− BMPC and peripheral blood PB/PC were analyzed for the expression of Ki-67 after cell fixation with formaldehyde and permeabilization with saponin. Frequencies of Ki-67+ cells are shown and were compared between blood PB/PC (6 samples, steady state) and BMPC subsets (Mann-Whitney U test; ***P < .001) and among pairs of BM CD19+ and CD19− PC (Wilcoxon test; *P < .05). Filled circles, Ki-67 staining (10 samples); open circles, control staining (available for 7 samples). Both BMPC subsets show frequencies of Ki-67+ cells significantly exceeding frequencies of isotype control stained cells (Mann-Whitney U test; both P < .01). Median values are indicated. (B) BM samples of 23 patients with rheumatoid arthritis treated with 2 × 1 g RTX (a chimeric anti-CD20 antibody) were analyzed for the presence of CD138+CD19+ and CD138+CD19− PC twice, once before, and once again 1 to 3 months after treatment. PC were detected by flow cytometry, and their numbers were extrapolated according to Trepel20 and were analyzed using the Wilcoxon test; *P < .05. Lines between data points indicate the trend shown by individual patients. Mean numbers of 3.10 × 108 (standard deviation [SD], 1.76 × 108) CD19+ and 1.36 × 108 (SD, 0.85 × 108) CD19− BMPC compare with 2.24 × 108 (SD, 1.69 × 108) CD19+ and 1.06 × 108 (SD, 0.93 × 108) CD19− BMPC after therapy, respectively.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/11/10.1182_blood-2014-02-555169/4/m_1739f4.jpeg?Expires=1769173468&Signature=OXfG9HgMnBUSQeSkHkEFalygo3CpeEMbUB-Ml-r9C5rJgLNZiv8Bc2gq7H--IEsly-hW6MujK18KSatDzjj3Uy5F0MRJFU2LlnBMopmjzxJEns1HKWff-EtWxGAfloD4yfFvubzkLwcfEAl4qeRv9zuHAiKrVTMCc4BMUpmB71S5tQS5otZB6HtvJzzyExGmAdRoOe371Mel27YoaC4Af-1sRwXLUE9KH6s~pS717PCA2LUbW18Fr4ShcBojUWsCOUA3C9n6FRGJb66EvDdtU~IIzIWyI3xQiBgZIfHfGX4G0LOkluxGzYs-Ujd3OYAXoboQb4JdsPuucHx6B0vC7Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal