In this issue of Blood, Tian et al report that during traumatic brain injury, small membrane vesicles, called microparticles, disseminate procoagulant factors from the brain into the systemic circulation. The delivery appears to occur through the disrupted blood-brain barrier.1
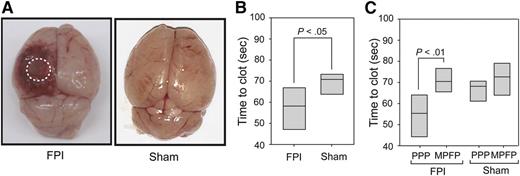
Trauma brain injury in mice and coagulation triggered by brain-derived microparticles. (A) Cerebral injury is visible in the left parietal lobe of a mouse 3 hours after induction of trauma using fluid percussion injury (FPI) compared with the brain from a mouse subjected to sham surgery (Sham). (B) The clotting time measured in platelet-poor plasma (PPP) collected from mice subjected to trauma brain injury (FPI) or sham surgery (Sham) was measured in a phosphatidylserine-dependent assay. (C) Clotting time was compared between PPP and homologous plasma depleted of any microparticles by centrifugation collected from mice subjected to trauma brain injury (FPI) and sham surgery (Sham). Adapted from Figure 1 in the article by Tian et al that begins on page 2151.
Trauma brain injury in mice and coagulation triggered by brain-derived microparticles. (A) Cerebral injury is visible in the left parietal lobe of a mouse 3 hours after induction of trauma using fluid percussion injury (FPI) compared with the brain from a mouse subjected to sham surgery (Sham). (B) The clotting time measured in platelet-poor plasma (PPP) collected from mice subjected to trauma brain injury (FPI) or sham surgery (Sham) was measured in a phosphatidylserine-dependent assay. (C) Clotting time was compared between PPP and homologous plasma depleted of any microparticles by centrifugation collected from mice subjected to trauma brain injury (FPI) and sham surgery (Sham). Adapted from Figure 1 in the article by Tian et al that begins on page 2151.
Defects in the coagulation process are common in patients with trauma, mostly due to major blood loss, consumption of coagulation factors, thrombocytopenia, fluid resuscitation, and hypothermia.2 As coagulopathy in trauma is associated with poor outcome, defining the factors that impair blood coagulation in this context is extremely important.
Intrigued by the coagulopathy that accompanies traumatic brain injury, a category of trauma generally not accompanied by heavy blood loss and fluid resuscitation,3 the authors hypothesized that factors generated by the injured brain could disseminate into the blood circulation and impact coagulation. Microparticles are extracellular vesicles measuring ∼0.1 to 1 µm in diameter. They are produced from activated and apoptotic cells by membrane blebbing and fission and can support coagulation through exposure of phosphatidylserine and tissue factor, which is the trigger of the extrinsic pathway of coagulation.4 Although the brain is rich in phosphatidylserine and tissue factor, nothing was known on the impact of brain-derived microparticles on coagulation. The authors thus surmised that microparticles produced in the brain could convey phosphatidylserine and tissue factor into the systemic circulation and thereby impact coagulation.
To verify this hypothesis, the authors modeled trauma brain injury in mice and verified the appearance of brain-derived microparticles in systemic circulation. They observed that concomitant with the arrival of brain-derived microparticles in blood, mice subjected to brain trauma injury developed a hypercoagulable state. The accelerated clotting time was due to microparticles, as their elimination by centrifugation abrogated the effect (see figure). To ensure that the microparticles they detected were indeed of brain origin and not produced in the periphery, the authors confirmed the expression of multiple neuronal and glial markers (neuron specific enolase, glial fibrillary acidic protein, Na+/K+ ATPase α3, and glutamate transporter-1). For comparison, they verified that microparticles shed from platelets, which represent the majority of the microparticles in blood at steady state and might also express a subset of neuronal proteins,5 were absent in this set of markers. As the brain-derived microparticles found in blood circulation expressed phosphatidylserine and tissue factor, one major finding from this study is that microparticles generated from brain injury can pass into the circulation.
Next, the authors went on to confirm the causal role of brain-derived microparticles in the modulation of coagulation. Isolated brain-derived microparticles generated in vitro were highly procoagulant due to surface phosphatidylserine and tissue factor expression. Another mechanism by which brain-derived microparticles may impact blood coagulation is through direct interaction with platelets. Platelets efficiently bound brain-derived microparticles, which triggered platelet activation. Notably, intravenous injection of brain-derived microparticles into uninjured mice led to fibrin deposition in the microvasculature and to fibrinogen consumption. Thus, consistent with the prolonged clotting time seen in trauma patients, the infusion of brain-derived microparticles in mice led to consumptive coagulopathy. Intriguingly, although the mice subjected to traumatic brain injury had a hypercoagulable state, those injected with brain-derived microparticles displayed coagulopathy. The rate at which the brain-derived microparticles were released into the circulation (over time in trauma vs bolus injection), possible qualitative differences between microparticles derived from the traumatic brain in vivo and those produced experimentally in vitro, and a potential contribution of microparticles produced by blood cells during trauma brain injury6 might explain these opposing observations.
One major question that arises is, How do microparticles egress the confined brain environment to reach the systemic circulation? The authors demonstrate that the blood-brain barrier is much more permeable after trauma, potentially facilitating the passage of small components, such as brain-derived microparticles, into the blood. To verify this, they modeled microparticle transmigration through an artificial endothelial barrier and observed that brain-derived microparticles could ramble through disrupted endothelial cell junctions. Activated platelets facilitated this process, possibly through histamine and serotonin, mediators already recognized as highly efficient at creating gaps between endothelial cells with dimensions compatible with those of microparticles.7 That the blood-brain barrier might permit the passage of extracellular vesicles is not new. In fact, other studies suggested that vesicles, biological or synthetic, in blood might invade the brain.8,9 What is novel and counterintuitive in this present study is that this process might not be unidirectional.
Thus, assuming that the mechanism of action of brain-derived microparticles, unveiled in this study, also occurs in humans with trauma brain injury, the authors suggest that the neutralization of the key actor phosphatidylserine, by annexin V or lactadherin, for instance, may have therapeutic value. Furthermore, these observations, which are important for the understanding of coagulopathy in traumatic brain injury, might also be relevant to other disorders in which the blood-brain barrier is more permeable and during which hemostasis is defective.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal