Abstract
Prophylactic application of clotting factor concentrates is the basis of modern treatment of severe hemophilia A. In children, the early start of prophylaxis as primary or secondary prophylaxis has become the gold standard in most countries with adequate resources. In adults, prophylaxis is reasonably continued when started as primary or secondary prophylaxis in childhood to maintain healthy joint function. Initial data support that adult patients with already existing advanced joint arthropathy benefit from tertiary prophylaxis with significantly lowered number of bleeds, almost complete absence of target joints, and less time off from work. Current prophylactic regimens, although very effective, do not completely prevent joint disease in a long-term perspective. Joint arthropathy in primary prophylaxis develops over many years, sometimes over a decade or even longer time periods. The ankle joints are the first and most severely affected joints in those patients and thus may serve in outcome assessment as an indicator of early joint arthropathy when followed by ultrasound or magnetic resonance imaging. Optimized outcome and best use of available resources is expected from individualization of therapy regimens, which comprises the individual’s bleeding pattern, condition of the musculoskeletal system, level of physical activity and the pharmacokinetic profile of the substituted coagulation factor, and most recently includes novel products with extended half-lives.
Prophylaxis in hemophilia
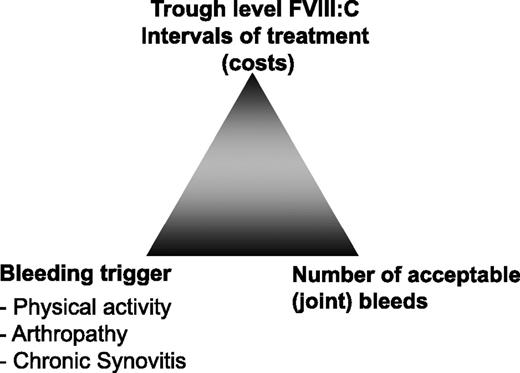
The key to a successful long-term outcome in patients with hemophilia is an efficient prophylaxis that prevents bleeding in joints for children and adults with hemophilia. Efficient prophylaxis requires taking into account the available resources (clotting factor concentrate, trough levels), the bleeding trigger (activity levels, chronic synovitis, already existing arthropathy), and most importantly the number of acceptable bleeds, especially joint bleeds (Figure 1). Depending on the available resources, the treatment objectives can vary between countries and treatment centers. In an almost ideal setting, the number of spontaneous bleeds should be minimized in order to prevent the manifestation of joint arthropathy. The severity of joint arthropathy mirrors as a kind of cumulative memory the number of experienced joint bleeds and, thus, reflects the overall quality of the prophylactic treatment regimen. Once joint damage has occurred, it will progress over the patient’s lifetime even if no further bleeds occur in the affected joints.1 As a consequence, primary prophylaxis should aim to prevent any joint damage. Early diagnosis of joint damage currently represents a challenge with routine imaging and clinical diagnosis tools. Moreover, joint arthropathy in a patient on primary prophylaxis develops very slowly, over a decade or even longer time periods. Both the subtle development of joint arthropathy and the limitations of its early detection hamper timely diagnosis and adequate action on treatment regimen.
The prophylaxis triangle. Prophylaxis treatment regimen has 3 main determinants: (1) the given resources/concentrate availability to target a specific trough level and/or intervals of substitutions, which both reflect the costs; (2) the bleeding trigger, which comprises physical activity, presence and degree of arthropathy, and presence of chronic synovitis; and (3) the number of bleeds, especially joint bleeds, that are regarded as acceptable. These 3 determinants form a triangle. If 1 determinant is changed, the other 2 will adjust. With unlimited resources, zero bleeds and normal physical activity may be targeted; with few resources, only 2 low-dose substitutions per week may be given, thus accepting a certain number of bleeds and limited physical activity.
The prophylaxis triangle. Prophylaxis treatment regimen has 3 main determinants: (1) the given resources/concentrate availability to target a specific trough level and/or intervals of substitutions, which both reflect the costs; (2) the bleeding trigger, which comprises physical activity, presence and degree of arthropathy, and presence of chronic synovitis; and (3) the number of bleeds, especially joint bleeds, that are regarded as acceptable. These 3 determinants form a triangle. If 1 determinant is changed, the other 2 will adjust. With unlimited resources, zero bleeds and normal physical activity may be targeted; with few resources, only 2 low-dose substitutions per week may be given, thus accepting a certain number of bleeds and limited physical activity.
Depending on the patient’s age and underlying conditions, prophylaxis and its subsequent prevention of bleeds have different objectives, which are reflected by the International Society on Thrombosis and Haemostasis (ISTH) definitions.2 According to these definitions, primary prophylaxis begins in early childhood in the absence of documented joint disease and before the second clinically evident joint bleed and before the age of 3 years. Patients treated in this way have the potential of a life without joint arthropathy. Secondary prophylaxis commences after 2 or more joint bleeds, but before the onset of joint disease as documented by physical examination and/or imaging studies. These patients may already have a significant risk of developing joint arthropathy. Tertiary prophylaxis is defined as treatment initiation after the onset of joint disease at any age of the patient. Objectives in those patients include slowing down progression of joint disease, reducing pain and inflammation, and maintaining mobility, especially in adult hemophiliacs with already advanced joint disease.
Although primary, and to a lesser extent secondary, prophylaxis represents the established gold standard for children in most developed countries, tertiary prophylaxis in adults is just recently becoming increasingly considered.
Current approaches improving prophylaxis outcome include best use of availablility of factor concentrate and individualization of treatment regimens with respect to dose, intervals, and type of concentrate, considering trough levels and bleeding triggers in the respective patients.
Initiation of prophylaxis in young children
Prophylaxis in children is most efficient when started at an early age. Prophylaxis initiated before the age of 2 years3 resulted in a better outcome than prophylaxis commenced at age 3 to 5 years or 6 to 9 years.4,5 National guidelines and recommendations in several European countries advise starting prophylaxis early.6,7 In the last decade, several studies suggest beginning with a once-weekly regimen.8-10 The rationale behind this is either to avoid the placement of central venous access devices and to account for the different clinical presentations, thus tailoring therapy and optimizing cost-effectiveness,8,9 or to lower the risk of inhibitor formation.10 The Canadian primary prophylaxis experience begins with a once-weekly regimen (50 U/kg body weight [BW]), that, depending on number of bleeds, is intensified in a first step to twice-weekly treatment (2 times 30 U/kg BW) and, in a third step, to every-other-day therapy (25 IU/kg BW).11 Median ages at switching to steps 2 and 3 were 4.1 and 9.7 years, respectively. Twenty percent of the patients remained on the lowest regimen during the observation time of this study. For this regimen, switches depend on the number of bleeds. Long-term follow-up observation of this cohort is needed, in order to assess whether this regimen is still effective against late onset of arthropathy. Kurnik et al presented data that suggest that early initiation of prophylaxis may prevent inhibitor formation.10 Triggered by observations from the CANAL and RODIN studies12,13 where inhibitor formation was significantly less in the prophylaxis group, compared with the on-demand group, Kurnik et al proposed beginning prophylaxis with once-weekly 25 IU/kg BW at a median age of 10 months.10 The rational behind this regimen was that regularly administered, low factor VIII (FVIII) doses in the absence of bleeds, intensive treatment periods, and danger signals may induce tolerance to FVIII within the first 20 to 50 exposure days. By the time the first major joint bleeds are expected, tolerance would already have been achieved. Following this regimen, Baxter commenced the prospective Early Prophylaxis Immunologic Challenge (EPIC) study. However, the EPIC study was stopped after inclusion of only 19 patients because of the unexpectedly high incidence of inhibitors. The reasons given for stopping the study were difficulties in adherence to the complex protocol in a multinational, multicenter study setting and an increasingly unrealistic perspective for achieving a significantly lower inhibitor frequency within this study.14
Dynamics of developing joint arthropathy
Current regimens are efficient, although they do not prevent joint arthropathy over a lifetime perspective
The onset of joint arthropathy largely depends on the number of bleeds per year, especially joint bleeds per year, which have become surrogate markers for outcome in both clinical and postmarketing studies. Although on-demand treated patients experience 20 to 50 bleeds per year and develop joint arthropathy early in life,1 patients treated prophylactically may remain free from joint disease over 1 to 2 decades or even longer. Manco-Johnson and coworkers performed the first controlled, randomized, and prospective trial comparing the onset of joint arthropathy in on-demand treated vs prophylactically treated young children.5 Joint disease was assessed by magnetic resonance imaging (MRI), an imaging tool allowing diagnosis of early joint damage. During an ∼4-year observation time, 45% of the on-demand group developed new joint arthropathy as opposed to only 7% in the prophylactic group. This study impressively demonstrated that prophylaxis is largely protective for joint disease over a 4-year period of observation. However, joint disease still occurred during this time in about 7% of the patients treated with an intensive prophylactic regimen (6000 IU/kg BW at the age of 6 years). Projected to a lifetime treatment, this means that at the age of 30 to 40 years, most hemophilia patients will suffer from some joint arthropathy.
So far, only a few studies have addressed long-term outcomes of prophylaxis in hemophilia patients with observation times ranging from 5 years to 2 to 3 decades.1,5,15-19 All studies support that, with the current treatment regimens, severe hemophilia patients will sooner or later develop joint arthropathy. Fischer et al compared The Netherlands’ intermediate-dose prophylaxis (2100 IU/kg per year) with the Swedish higher-dose prophylaxis regimen (4000 IU/kg per year).19 After a median observation time of 20 years at an age of young adulthood between 19 and 30 years, the intermediate prophylaxis regimen showed a median Hemophilia Joint Health Score (HJHS) of 7 (range, 2-18) of 144, whereas the Swedish group presented a median HJHS of 4 (range, 2-6.8). The number of joint bleeds in the preceding 5 years in the groups were 2 per year vs 0.5 per year, respectively.19 The Swedish protocol had a slightly better outcome and the difference between the 2 cohorts is expected to become larger with time. However, even the high-dose Swedish protocol, with only 1 joint bleed every 2 years, did not entirely prevent joint arthropathy over a lifetime perspective. Furthermore, it has to be taken into account that the summarized results were mainly clinical scores which are indicating joint disease later than MRI.5 A 25-year follow-up of 30 Swedish patients confirmed that early initiation of primary prophylaxis continuing throughout life was successful in virtually eliminating joint bleeds and preserving an acceptable, however not normal, joint status.18 In a 1992 study, Brackmann et al followed 90 German patients with severe hemophilia A from 1978 to 1990, and found no worsening of the joint status over a 12-year period.16 A 26-year follow-up of 49 patients of the same cohort, however, demonstrated that 90% of these patients treated throughout life with an intensive prophylactic regimen exhibited joint disease at 30 to 40 years of age.20
The ankle joint as arthropathy indicator
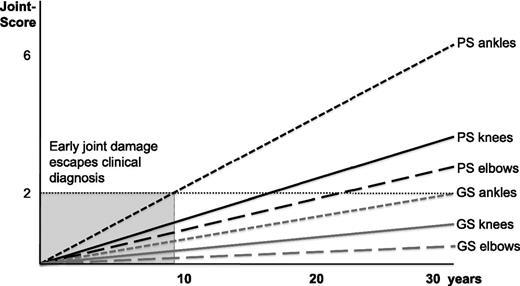
Before the era of prophylaxis (eg, in the 1970s), knee joint bleeds were the most frequently noted joint bleeds and knees were the clinically leading joint in most patients.21 With the beginning of the era of prophylaxis, the knee joint, as a muscle-controlled joint, became more stable and the ankle joints became the first joints to be affected and, thus, represent the clinically most indicative joint. Recently, Oldenburg et al observed higher MRI scores for ankle joints, and the scarcity of pathologic findings in knees for patients with unaffected ankles indicated that, in most patients, the ankle was the primary target joint of hemophilic arthropathy.22 These results are consistent with previous reports showing more hemarthroses and worse joint scores for ankles, compared with knees or elbows.5,23-26 Krämer et al’s 2013 study20 followed the development of joint arthropathy in the cohort on intensive lifelong prophylaxis, initially described by Brackmann et al16 in their article in 1992. During a 26-year follow-up, Pettersson scores based on plain x-rays were taken every 3 to 5 years and Gilbert scores were obtained once per year. Ankles were the first joints to be found affected by Pettersson score, with median pathologic findings after 10 years, followed by significant effects for knee and elbow joints after about another 10 years.20 On average, the clinical Gilbert scores became pathologic about 1 to 2 decades later than the Pettersson scores.20 An abstraction/simplification of the findings from Krämer in 2013 is illustrated in Figure 2. Our study as well as those by other authors indicate that imaging score-based diagnosis of arthropathy is possible long before being revealed by clinical scores.5 However, plain x-ray is too insensitive to diagnose early joint pathology, making it inappropriate to diagnose early joint damage. Ankles, as the first affected joints for the majority of patients, offer an interesting opportunity for diagnosing early joint disease and subsequently adapting clinical decisions.
Illustration and schematization of the long-term outcome results. Results of the 2013 study by Krämer et al,20 underlining that progression of joint arthropathy during intensive prophylaxis regimens is a process with subtle progression over years. Scores ≥2 are regarded to be pathological. The ankle joints are the first joints that develop arthropathy after a median time of 10 years, followed by knee joints and elbow joints significantly later. The clinical Gilbert scores are following the Pettersson scores 1 to 2 decades later. The gray area indicates the initial decade of prophylactic treatment when early joint disease remains undetected by the Pettersson score. GS, Gilbert score; PS, Pettersson score.
Illustration and schematization of the long-term outcome results. Results of the 2013 study by Krämer et al,20 underlining that progression of joint arthropathy during intensive prophylaxis regimens is a process with subtle progression over years. Scores ≥2 are regarded to be pathological. The ankle joints are the first joints that develop arthropathy after a median time of 10 years, followed by knee joints and elbow joints significantly later. The clinical Gilbert scores are following the Pettersson scores 1 to 2 decades later. The gray area indicates the initial decade of prophylactic treatment when early joint disease remains undetected by the Pettersson score. GS, Gilbert score; PS, Pettersson score.
Outcome assessment
Prophylaxis has greatly improved joint health and is challenging joint outcome assessment. Because of the low number of about 1 joint bleed every 2 years resulting from intensive prophylaxis programs,5,19,20 clinical manifestation of joint disease has become a subtle process, starting mildly, subclinically, and progressing slowly over years. Thus, diagnosing early joint disease and taking timely and adequate action has become difficult.
Outcome assessment comprises several tools including annual bleed rates, physical joint examination, and imaging measures such as plain x-rays, MRI, and ultrasound assessment. Quality-of-life questionnaires complement the instrumentarium for outcome assessment (as discussed by Blanchette et al27 in 2014). The HJHS is currently regarded as the state-of-the-art instrument for clinical assessment of joint status. It includes most of the elements of the previously used Gilbert score.28-31 Plain x-rays scored by the Pettersson scale had been a useful tool in the past, but are not able to recognize early joint disease.16,32 MRI is a very sensitive instrument for early detection of hemarthrosis, including synovial hypertrophy, hemosiderin deposition, and osteochondral changes. Scoring scales for reliable assessment of hemophilia arthropathy have been established.33-36 However, MRI application to young children is limited and also expensive. More recently, ultrasound has been used for diagnosis of joint disease in hemophiliacs. Advantages are its wide availability and its low cost. Soft-tissue changes such as synovial hypertrophy, which precede joint disease, especially can be effectively diagnosed. Standardized and simplified ultrasound scanning protocols for early arthropathy detection in ankles, knees, and elbows have been published.37-40 The joint measures for long-term outcome with respect to established scores, potency of detecting early joint disease and follow-up of severe arthropathy, suggested intervals for assessment, and costs are summarized in Table 1.
Joint outcome measures for long-term follow-up
| . | Established scores . | Detection of early joint disease . | Follow-up of severe arthropathy . | Suggested intervals for outcome measure . | Costs . |
|---|---|---|---|---|---|
| HJHS | +++ | — | +++ | Annually (ankles, knees, elbows) | Low |
| Plain x-ray (Pettersson score) | +++ | — | +++ | Every 5 y (ankles, knees, elbows) | Low |
| MRI | +++ | +++ | (+++) | Every 5 y starting at age of 6-8 y (ankles) | Very high |
| Ultrasound | ++ | ++ | — | Annually (ankles, knees, elbows) | Low |
| . | Established scores . | Detection of early joint disease . | Follow-up of severe arthropathy . | Suggested intervals for outcome measure . | Costs . |
|---|---|---|---|---|---|
| HJHS | +++ | — | +++ | Annually (ankles, knees, elbows) | Low |
| Plain x-ray (Pettersson score) | +++ | — | +++ | Every 5 y (ankles, knees, elbows) | Low |
| MRI | +++ | +++ | (+++) | Every 5 y starting at age of 6-8 y (ankles) | Very high |
| Ultrasound | ++ | ++ | — | Annually (ankles, knees, elbows) | Low |
Joint outcome measures for long-term follow-up with respect to established scores, potency of detecting early joint disease, and follow-up of severe arthropathy, suggested intervals for assessment, and costs.
+++ indicates very good; —, no or limited value; ++, good.
However, the interrelationships among these scores have not been established and validated. A potential future perspective to follow joint health status might start with annual physical and ultrasound scoring in young children and, at the age of 6 to 8 years, an initial MRI, preferably of both ankle joints. Further studies are needed that compare these scores over time and comparatively assess their applicability. Quality-of-life scales support the benefits of prophylaxis with the aim of an almost normal lifelong health.2
The annual bleed rate (ABR) has become an important parameter in clinical studies as a surrogate for the efficiency of prophylaxis regimens. Some studies have also assessed the annual joint bleed rate. For intensive treatment protocols, the mean total ABRs range from 2 to 5, whereas the joint bleed ABR is in the order of 0.5.5,20,41-43 Given the long-term dimension of developing joint arthropathy and the limitations of diagnosing early joint damage the ABR serves as an important clinical parameter to adjust treatment regimens.
Prophylaxis in adult patients
Although primary prophylaxis represents the gold standard for preserving joint function in children with severe hemophilia, prophylaxis in adult patients is still debated. There are 2 groups of adult patients who have to be addressed separately. The first group of patients includes those who started primary or secondary prophylaxis early in their life and maintained good joint health into adulthood. A small number of published studies suggest that these patients benefit from lifelong prophylaxis.16,18-20 These studies reported a follow-up of 12 to 30 years and demonstrated that hemophilia patients with an ongoing prophylaxis regimen present with well-preserved joint function and only mild joint arthropathy at the age of 30 to 40 years.18-20 The patients from The Netherlands with an intermediate dosage regimen had a slightly worse outcome at the age of 30 years than Swedish patients with a high-dose prophylaxis regimen.19 In the German cohort, 90% of patients showed some, mostly mild, arthropathy, mainly in their ankle joints after a 26-year follow-up.20 There are several reports that a proportion of young adults are switched to on-demand regimens and that some of these patients experience only slight bleeding and little joint arthropathy.44,45 However, no follow-up data are available that report joint condition of these patients at the age of 40 to 50 years or older. As initial joint damage always progresses, even in the absence of joint bleeds, a worsening of joint disease can be anticipated. The joint annual bleed rate might serve as a surrogate for switching those patients back to a prophylactic regimen. The joint ABR should not be higher than during an intensive prophylaxis regimen, which is about 1 to 2 joint bleeds within 2 years.
The second group of adult hemophilia patients are those who already present with an advanced joint arthropathy and are on a tertiary prophylactic regimen. There are few studies that impressively demonstrate the reduction of total bleeds and joint bleeds in patients who are on tertiary prophylaxis compared with an on-demand regimen. The prospective randomized Trial to Evaluate the Effect of Secondary Prophylaxis with rFVIII Therapy in Severe Hemophilia A Adult and/or Adolescent Subjects Compared to That of Episodic Treatment (SPINART study) found a median of about 54.5 total bleeds per year in patients treated on-demand compared with a median of 0 total bleeds in the prophylactic group.41 Interestingly, about 20% of the prophylaxis group, with 25 IU/kg BW 3 times per week, still had a significant number of bleeds, indicating the need for further individual adaptation of the prophylaxis treatment. A recent survey in Europe examined the use of prophylaxis in people aged 20 to 35 years with severe hemophilia and found an inverse correlation between time on prophylaxis and occurrence of major bleeds, presence of target joints and time off from work. Patients from Sweden who spent the longest period on prophylaxis had the best preserved joints and best quality of life.46
A cross-sectional MRI evaluation of joint status in severe hemophilia A patients treated with prophylaxis initiated at different ages vs on-demand therapy demonstrated protective effects of prophylaxis. All prophylaxis groups had better MRI joint scores than the on-demand group. MRI scores generally increased with current patient age and later start of prophylaxis. Ankles were the most affected joints.22 These results also indicate that adults with already established severe joint arthropathy benefit from a prophylactic regimen in terms of number of bleeds, presence of target joints, mobility, and time off from work. However, long-term follow-up studies are needed to substantiate these effects.
Individualizing of treatment regimens
Individualization of therapy would not be an issue if the trough factor levels could be raised in every patient to 15% to 20%, thus allowing an almost bleed-free life. However, resources are limited and individualization is applied to get the best outcome with the given resources. On an economic basis, individualization also implicates that a certain risk of bleeding is taken. An individualized regimen comprises the individual’s bleeding pattern, the condition of the musculoskeletal system, level and timing of physical activity, and actual levels as well as trough levels of coagulation factor.47
Individual bleeding risk: more than factor levels
A subset of 10% to 15% of patients with severe hemophilia A exhibits a mitigated disease phenotype, with significantly reduced frequencies of spontaneous bleeding and lower consumption of factor concentrates.48 This clinical heterogeneity is also reflected by the late onset of the first joint bleed and furthermore in development of only minimal arthropathy. This mitigated clinical phenotype is chiefly determined by the underlying mutations in the F8/F9 genes.49,50 Missense mutations, splice site mutations outside conserved regions, and small deletions within A series were especially associated with less bleeding.51 Furthermore, the inflammatory response to the presence of blood in a joint varies, which is believed to be in part dependent on genetic variations in the genes involved in the inflammatory and immune-regulatory pathways.52-54 This variation may influence the subsequent development of chronic synovitis and also, ultimately, joint arthropathy. Indeed, the Joint Outcome Study reported several boys with multiple episodes of hemarthrosis who remained free of joint damage. Other patients showed joint damage in the absence of clinical bleeds.5 In patients with greater numbers of bleeds or with a stronger inflammatory response to a bleed, larger factor doses and more frequent application are required to prevent the onset of joint disease.
Pharmacokinetic variation in patients and new products
Collins et al demonstrated a great variation of pharmacokinetics not only dependent on age (shorter half-life in young children than in adults) but also within patient groups of the same age, where he found an almost 100% difference in time-to-trough levels of >1% after application of a standardized FVIII dose.55 Several studies have shown that the level of the VWF has a major influence on the FVIII half-life.56-58 Higher VWF levels correlate with increasing intervals of FVIII substitutions.57,58 Therefore, assessment of the patient’s individual pharmacokinetic profile is regarded as important for individualization of therapy. Because a classical pharmacokinetic profile is based on numerous blood draws over 2 or more days, population pharmacokinetics presents an elegant approach to assess the pharmacokinetic profile by routine factor level measurements at regular visits in the treatment center.59,60 Population pharmacokinetics will become an important measure for individualized treatment regimens.60
A number of new factor concentrates and drugs based on other technologies with improved half-lives and alternative administration routes will soon be available.61 The advances for recombinant factor IX (FIX) products have been significant, with half-life extensions up to 100 hours, allowing substitution intervals of 1 to 2 weeks. For recombinant FVIII products, the advances thus far are only moderate, as the half-life extension is limited to about 15 to 18 hours by the clearance of FVIII through VWF.61 Using longer-acting coagulation factors with different pharmacokinetic profiles will further individualize treatment by maintaining adequate trough levels with fewer infusions.47 On the horizon are novel products applying new technologies such as a bispecific antibody that mimics FVIII.61,62 This product has the potential to almost eliminate bleeds by weekly subcutaneous injections of the bispecific antibody.63
Prophylaxis in hemophilia B
There is an ongoing discussion about whether the phenotypes of hemophilia A and hemophilia B have the same phenotype or whether hemophilia B patients have fewer bleeds and develop joint disease later and less severely.64,65 The mutation spectrum varies significantly. Although in hemophilia A about 80% of the patients exhibit null mutations with no endogenous FVIII protein synthesis, hemophilia B show only about 20% to 30% null mutations.66,67 Hemophilia B especially is much more commonly caused by missense mutations, which might be associated with some small amounts of endogenous plasma FIX protein. In their 2010 study, Santagostino and coworkers showed that the type of mutation was the only significant parameter influencing the phenotype.49 Although this different mutation profile is explaining the different inhibitor incidence,68 it is not clear whether it may also affect the treatment regimens in hemophilia B patients. Most recently, Clausen et al reported a total of 582 patients with severe hemophilia A and 76 with severe hemophilia B from the RODIN study and found that hemophilia A and hemophilia B did not differ in age at first exposure to clotting factor, age at first bleed, and age at first joint bleed.69 Although these data refer to a very early stage of treatment, decisions on the prophylactic treatment are made at this time and are based on criteria such as the onset of bleeds, especially joint bleeds. Therefore, it appears safe to follow the same principles for prophylaxis in hemophilia B patients as has been outlined in this review for hemophilia A patients. The longer half-life of FIX and especially the new recombinant FIX products with extended half-lives of up to 100 hours will make a difference in the prophylactic regimens.
Conclusion
In conclusion, current therapy regimens include early start of prophylaxis as primary or secondary prophylaxis. Prophylaxis is increasingly regarded as a lifelong therapy, also applied as tertiary prophylaxis in adults with already existing arthropathy. There is a trend toward a more intensive prophylaxis, achieving an almost quantitative prevention of joint bleeds. Individualized strategies intend to optimize outcome and utilization of resources. Current data are limited to a follow-up period of a maximum of 25 to 30 years. There are no data available demonstrating outcomes in a lifetime perspective. Future studies are needed to provide long-term outcome data for current regimens. The parallel development of gene therapy protocols that are already in place for hemophilia B and that are at the horizon for hemophilia A may result in a cure for hemophilia patients, at least with respect to spontaneous bleeds.70 At the time of clinical availability of gene therapy, the risks and benefits, also with respect to possible late adverse events, have to be balanced against the classical prophylaxis regimens with the new products available at that time.
Authorship
Contribution: J.O. designed the review, studied the literature, and wrote the manuscript.
Conflict-of-interest disclosure: J.O. received reimbursement for attending symposia/congresses, and/or honoraria for speaking, and/or honoraria for consulting, and/or funds for research from Baxter, Bayer, Biogen Idec, Biotest, CSL-Behring, Grifols, Novo Nordisk, Octapharma, Swedish Orphan Biovitrum, and Pfizer.
Correspondence: Johannes Oldenburg, Institute of Experimental Haematology and Transfusion Medicine, University Clinic Bonn, Bonn, Germany; e-mail: johannes.oldenburg@ukb.uni-bonn.de.