Key Points
The combination of intratumoral CpG with systemic ibrutinib results in complete and permanent regression of both local and distant tumors.
The antitumor effect of the combination is T-cell dependent.
Abstract
We have designed a novel therapeutic approach for lymphoma that combines targeted kinase inhibition with in situ vaccination. Intratumoral injection of an unmethylated cytosine guanine dinucleotide (CpG)-enriched oligodeoxynucleotide, an agonist for the toll-like receptor 9 (TLR9), induces the activation of natural killer cells, macrophages, and antigen presenting cells that control tumor growth at the local site. Ibrutinib, an irreversible inhibitor of Bruton’s tyrosine kinase, a key enzyme in the signaling pathway downstream of B-cell receptor, is an effective treatment against many types of B-cell lymphomas. The combination of intratumoral injection of CpG with systemic treatment by ibrutinib resulted in eradication of the tumors not only in the injected site, but also at distant sites. Surprisingly, this combinatorial antitumor effect required an intact T-cell immune system since it did not occur in nude, severe combined immunodeficiency, or T-cell depleted mice. Moreover, T cells from animals treated with intratumoral CpG and ibrutinib prevented the outgrowth of newly injected tumors. This result suggests that ibrutinib can induce immunogenic cell death of lymphoma cells and that concomitant stimulation of antigen-presenting cells in the tumor microenvironment by toll-like receptor ligands can lead to a powerful systemic antitumor immune response.
Introduction
Single stranded, unmethylated, cytosine guanine dinucleotide (CpG) oligodeoxynucleotides can mimic bacterial DNA.1,2 These DNA sequences stimulate antigen-presenting cells (APCs) through their intracellular toll-like receptor 9 (TLR9).3 Systemic antitumor immune responses can be achieved if CpG oligodeoxynucleotide is injected directly into one local tumor site where tumor cells dying as a result of chemotherapy or radiotherapy release their antigens. This combination of local immunotherapy and tumor cell death evokes a CD8 T-cell–mediated immune response that can eradicate tumors throughout the animal.4 Another potent systemic immunotherapy can be accomplished without the use of cytotoxic chemo/radiotherapy, by combining intratumoral CpG with antibodies that deplete T-regulatory cells in the tumor microenvironment.5
Ibrutinib is an irreversible inhibitor of Bruton’s tyrosine kinase (BTK), a critical member of the B-cell receptor-signaling pathway.6-9 BTK is important for the development and maintenance of malignant and normal B cells. Ibrutinib has dramatic antitumor effects in chronic lymphocytic leukemia (CLL) and other B-cell malignancies.10-15 However, this drug also inhibits other members of the Tec family of tyrosine kinases, such as IL-2–inducible T-cell kinase (ITK), an important member of the signaling pathway in T cells, especially the Th2 subset of CD4 T cells.6,8,9,16 Hence, ibrutinib can shift the balance of T-cell responses toward the more therapeutically effective Th1 subset.
Therefore, we tested whether ibrutinib, both a killer of malignant B cells and a potential enhancer of T-cell immune responses, could augment the therapeutic effect of intratumoral CpG. We found this powerful combination to be therapeutically effective, providing a novel, safe, and practical form of immunotherapy.
Materials and methods
Reagents
CpG 1826 5′-TCCATGACGTTCCTGACGTT with phosphothioate backbone was provided by Pfizer Vaccines Research (Ottawa, Ontario, Canada). Ibrutinib was provided by Pharmacyclics Inc. (Sunnyvale, CA). Anti-mouse CD8a (clone 2.43) and anti-mouse CD4 (clone GK1.5) antibodies were purchased from Bio X Cell (West Lebanon, NH). The isotype control rat hybridoma, SFR8-B6 (ATCC HB-152), was produced as ascites in severe combined immunodeficiency (SCID) mice by Bionexus (Oakland, CA).
The following monoclonal antibodies (mAbs) were used for flow cytometry: rat anti-mouse CD4- PerCP cy5.5, rat anti-mouse CD3- PerCP cy5.5, rat anti-mouse CD8a-fluorescein isothiocyanate, rat anti-mouse CD44-APC, rat anti-mouse interferon (IFN)-γ-PE. These antibodies and their isotype controls were purchased from either BD Biosciences or eBioscience.
Cell lines and mice
The H11 pre–B-cell line was generated from a C57BL/6 mouse as previously described.4 BL3750 is a murine C57BL/6 B-cell line from a cMyc transgenic mouse.17 A20, a B-cell lymphoma line, was obtained from American Type Culture Collection (Manassas, VA). Tumor cells were cultured in complete medium (RPMI 1640; Cellgro) containing 10% fetal bovine serum (HyClone), 100 U/mL penicillin, 100 µg/mL streptomycin, and 50 µM 2-ME (Gibco).
Six- to 8-week-old female BALB/C mice were purchased from the Jackson Laboratory (http://jaxmice.jax.org/), and C57BL/6 and Fox Chase SCID (CB17/Icr-Prkdcscid/IcrIcoCrl) female mice were purchased from Charles River (http://www.criver.com). Mice were housed in the Laboratory Animal Facility of the Stanford University Medical Center (Stanford, CA). All experiments were approved by the Stanford University administrative panel on laboratory animal care, and conducted in accordance with the Stanford University animal facility guidelines.
Tumor inoculation and animal studies
H11, A20, and BL3750 tumor cells (1 × 106, 5 × 106, and 1 × 106, respectively) were injected subcutaneously at sites on both right and left of the abdomen. CpG was injected into the tumor only on the right side on days 8 to 12 at 100 µg/dose in a volume of 50 µL.
Ibrutinib was injected by the intraperitoneal route at a dose of 6 mg/kg beginning on day 8 after tumor implantation and continued daily for a total of 8 days. Tumor size was monitored on both sides of the animals with a digital caliper (Mitutoyo) every 2 to 3 days and expressed as volume (length × width × height). Mice were euthanized when tumor size reached 1.5 cm in the largest diameter as per guidelines.
Flow cytometry
Cells were surface stained in phosphate-buffered saline, 1% fetal bovine serum, and 0.01% sodium azide, fixed in 2% paraformaldehyde, and analyzed by flow cytometry on an FACSCalibur (BD Biosciences). Data were stored and analyzed using Cytobank (www.cytobank.org).
Statistical analysis
Prism software (GraphPad Software, La Jolla, CA) was used to analyze tumor growth and to determine statistical significance of differences between groups by applying an unpaired Student t test. P values < .05 were considered significant.
In vitro assays
Growth and viability of cells cultured in 96-well plates was measured by using PrestoBlue Cell Viability Reagent (Life Technologies) according to the manufacturer’s instructions.
IFN-γ production assay
Single-cell suspensions were made from spleens of treated mice and red cells were lysed with ammonium chloride, potassium buffer (Quality Biological, Gaithersburg, MD). Splenocytes were then cocultured with 1 × 106 irradiated H11 or BL3750 cells for 24 hours at 37°C and 5% CO2 in the presence of 0.5 µg anti-mouse CD28mAb (BD Pharmingen). Monensin (GolgiStop; BD Biosciences, San Jose, CA) was added for the last 5 to 6 hours. Intracellular IFN-γ expression was assessed using BD Cytofix/Cytoperm Plus Kit per the manufacturer’s instructions.
Depletion of CD4 and CD8 T cells
Anti-CD4 (GK1.5 clone- rat IgG2b) and anti-CD8 (2.43 clone- rat IgG2b) mAbs (Bio X Cell) were injected 2 days and 1 day before therapy, on the day therapy was begun, and at 5, 8, and 19 days after beginning of therapy, at a dose of 0.5 or 0.1 mg per injection for CD4 and CD8, respectively. The depletion conditions were validated by flow cytometry of blood, showing specific depletion of more than 95% of each respective cell subset.
Adoptive immunity transfer experiments: Winn assay
Spleen cells were taken from mice 5 days after the beginning of therapy, at a time when tumors were regressing. Total T cells (CD3), CD4, or CD8 T cells were isolated by negative selection using cell isolation kits (Dynabeads, Life Technologies). CD3, CD4, and CD8 cells purity was assessed by flow cytometry and confirmed to be > 95%. These T cells were mixed with H11 tumor cells at a ratio of 100:1 and co-injected into naïve C57BL/6 mice. Tumor growth in the recipient mice was measured as described above.
Results
Sensitivity of B-lineage tumor cells to ibrutinib and to CpG
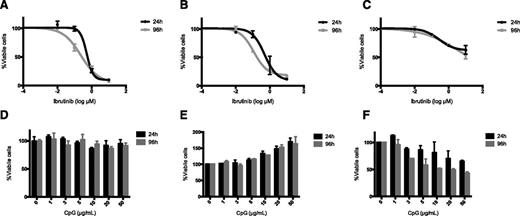
We chose several cell lines to test the interaction of ibrutinib and CpG. H11 is a pre–B-cell tumor from a C57/BL6 mouse,18 BL3750 is a mature B-cell lymphoma from a cMycTG+/− C57/BL6 mouse,17 and A20 is a mature B-cell lymphoma from a BALB/C mouse. Cells from these 3 tumors were treated in vitro with ibrutinib at concentrations ranging from 0.04 to 10 µM (Figure 1A-C) and stained for cell viability using PrestoBlue. Growth of both H11 and BL3750 cells was inhibited by ibrutinib (IC50 0.2 to 0.6 µM). This level of ibrutinib sensitivity was comparable to that of primary human CLL tumor cells.19,20 By contrast, A20 tumor cells were insensitive to ibrutinib (IC50 >10 µM; Figure 1C). Similar IC50 values were obtained when fresh ibrutinib was introduced daily. CpG had no effect on the growth of H11, however, it promoted survival of the BL3750 cells and it had an inhibitory effect on the growth of A20 tumor cells, as previously described4 (Figure 1D-F).
Cell survival following ibrutinib and CpG treatment. (A,D) H11, (B,E) BL3750, and (C,F) A20 cells were incubated with serial dilutions of ibrutinib or CpG at concentrations ranging from 0.04 to 10 µM of ibrutinib and 1 to 50 µg/mL of CpG, or with media alone for the indicated time. Cell survival was measured by PrestoBlue staining. (A-C) Ibrutinib; (D-F) CpG.
Cell survival following ibrutinib and CpG treatment. (A,D) H11, (B,E) BL3750, and (C,F) A20 cells were incubated with serial dilutions of ibrutinib or CpG at concentrations ranging from 0.04 to 10 µM of ibrutinib and 1 to 50 µg/mL of CpG, or with media alone for the indicated time. Cell survival was measured by PrestoBlue staining. (A-C) Ibrutinib; (D-F) CpG.
From these data, we reasoned that ibrutinib might be useful for therapy against H11 and BL3750, and that CpG might act on both the tumor cells and the host APCs to evoke an immune response to antigens released by dying tumor cells.
The combination of intratumoral CpG and systemic ibrutinib induces regression of local and distant tumors
The intratumoral injection of CpG can activate local natural killer (NK) cells, macrophages, and dendritic cells (DCs) and thereby cause the regression of a tumor at that injected site. However, this alone does not induce a therapeutically effective systemic antitumor immune response. We hypothesized that ibrutinib would kill tumor cells, release tumor antigens to be taken up by the CpG-activated APCs, that would in turn, induce a more potent T-cell immune response.
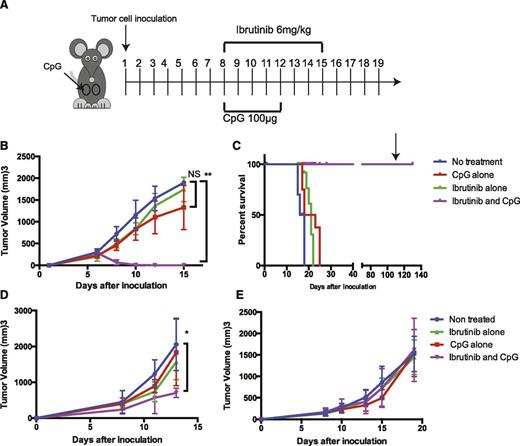
To test this hypothesis, mice were inoculated with H11 cells at 2 different subcutaneous sites (left and right sides of the abdomen). After the tumors were established and had reached a size of 0.7 to 1 cm in diameter, usually by day 8 (Figure 2A), the mice were treated with ibrutinib alone, CpG alone (each at a dose that maximized their independent therapeutic effect), or the combination of ibrutinib and CpG. Only the right tumor was injected with CpG and the tumor on each side of the body was monitored for growth. As expected, intratumoral injection of CpG led to regression at the injected site, but had no effect on the growth of the tumor at the nontreated site. Systemic ibrutinib treatment induced a slight delay in tumor growth at both tumor sites. In contrast, the combination of intratumoral CpG to the right tumor with systemic ibrutinib resulted in complete and permanent regression of tumors on both sides of the animals (Figure 2B-C). Surviving mice were resistant to rechallenge 120 days later with a double dose of H11 tumor cells, whereas the tumor grew as expected in the control naïve mice.
Ibrutinib plus intratumoral CpG induces a systemic antitumor effect in ibrutinib-sensitive tumors. C57BL/6 mice were inoculated with 1 × 106 H11 or BL3750 cells and BALB/C were inoculated with 5 × 106 A20 cells subcutaneously on both right and left of their abdomen. Tumor growth was monitored with a digital caliper. Mice were treated with ibrutinib/CpG alone or ibrutinib plus CpG. Ibrutinib (6 mg/kg) was given daily from day 8 after tumor implantation for 8 days. CpG (100 µg) was injected daily into the right tumor on days 8 to 12. (A) Schema of treatment. (B) The average tumor size of the nontreated left tumor. (C) Kaplan–Meier survival curves. On day 120, surviving mice were rechallenged with 2 × 106 H11 cells. (D-E) The average tumor size of the nontreated left tumor BL3750 and A20, respectively. Statistically significant differences (indicated by asterisks); P < .05. Each tumor model included n = 10 mice. Three separate experiments with similar results were performed. Error bars indicate SD. NS, not significant.
Ibrutinib plus intratumoral CpG induces a systemic antitumor effect in ibrutinib-sensitive tumors. C57BL/6 mice were inoculated with 1 × 106 H11 or BL3750 cells and BALB/C were inoculated with 5 × 106 A20 cells subcutaneously on both right and left of their abdomen. Tumor growth was monitored with a digital caliper. Mice were treated with ibrutinib/CpG alone or ibrutinib plus CpG. Ibrutinib (6 mg/kg) was given daily from day 8 after tumor implantation for 8 days. CpG (100 µg) was injected daily into the right tumor on days 8 to 12. (A) Schema of treatment. (B) The average tumor size of the nontreated left tumor. (C) Kaplan–Meier survival curves. On day 120, surviving mice were rechallenged with 2 × 106 H11 cells. (D-E) The average tumor size of the nontreated left tumor BL3750 and A20, respectively. Statistically significant differences (indicated by asterisks); P < .05. Each tumor model included n = 10 mice. Three separate experiments with similar results were performed. Error bars indicate SD. NS, not significant.
A similar, although less dramatic effect was seen with the ibrutinib-sensitive BL3750 tumor (Figure 2D) but not with the ibrutinib-insensitive A20 tumor (Figure 2E). These results were consistent with our hypothesis of direct tumor killing by ibrutinib, followed by a systemic immune response triggered by antigen presentation at the site of CpG injection leading to systemic antitumor response.
Tumor regression is dependent on CD4 and CD8 T cells
Systemic tumor regression mediated by the combination of intratumoral CpG and systemic ibrutinib occurred as soon as 4 days after the initiation of treatment. The speed of this response suggested involvement of the innate, rather than the adaptive, immune system. To test this hypothesis, we performed the same combination experiment in SCID mice that were devoid of T and B cells but replete in myeloid-derived cells and NK cells. Surprisingly, in these mice, the combination of CpG and ibrutinib failed to cause tumor regression either at the local site or at the noninjected site (Figure 3A). This result suggested that T cells and/or B cells were required for therapeutic effect of ibrutinib and intratumoral CpG.
The therapeutic effect of combination of ibrutinib plus CpG is T cell dependent. Mice were inoculated with H11 cells and treated with ibrutinib and intratumoral injection of CpG. Tumor outgrowth of (A) SCID mice, (B) nude mice, (C) T-cell depleted wild-type C57BL/6 mice (CD4/CD8) or both T cells were depleted by IP injection of anti-CD4 (GK1.5 clone) or anti-CD8 T cells (2.43 clone). Complete depletion of the CD8 or the CD4 T-cell populations was confirmed by flow cytometry of peripheral blood. Abs were given days 3, 2, 1, and 0 before treatment and the depletion was maintained throughout the experiment. Groups included: isotype control, CD4 T-cell–depleted, CD8 T-cell–depleted, and CD8- and CD4-cell–depleted mice. Each line represents the average left, nontreated tumor measurement of 10 mice. Two separate experiments with similar results were performed. Error bars indicate SD. NS, not significant.
The therapeutic effect of combination of ibrutinib plus CpG is T cell dependent. Mice were inoculated with H11 cells and treated with ibrutinib and intratumoral injection of CpG. Tumor outgrowth of (A) SCID mice, (B) nude mice, (C) T-cell depleted wild-type C57BL/6 mice (CD4/CD8) or both T cells were depleted by IP injection of anti-CD4 (GK1.5 clone) or anti-CD8 T cells (2.43 clone). Complete depletion of the CD8 or the CD4 T-cell populations was confirmed by flow cytometry of peripheral blood. Abs were given days 3, 2, 1, and 0 before treatment and the depletion was maintained throughout the experiment. Groups included: isotype control, CD4 T-cell–depleted, CD8 T-cell–depleted, and CD8- and CD4-cell–depleted mice. Each line represents the average left, nontreated tumor measurement of 10 mice. Two separate experiments with similar results were performed. Error bars indicate SD. NS, not significant.
To test the requirement for B cells, we repeated the experiment in nude mice lacking functional T cells but replete in B cells. Once again, the therapeutic synergy between ibrutinib and intratumoral CpG did not occur in these mice, implying that T cells, rather than B cells, were required for this effect (Figure 3B).
To further evaluate which of the T-cell populations is required for the tumor regression, we repeated the experiment in immune-competent mice and used antibodies to specifically deplete CD4, CD8, or both. The mice that were not treated with the depleting antibodies and those receiving the isotype control antibody responded to the combined treatment with tumor regression. Depletion of CD4, CD8, or both, abolished the therapeutic effect (Figure 3C).
From this series of experiments, we conclude that both CD8 and CD4 T cells, but not B cells and NK cells, are required for an effective antitumor response by this maneuver.
Depletion of T cells (Figure 3) examines the induction of immunity. Next, we sought to investigate the effector phase of immunity and the causal role of T cells in resisting the tumor growth. We isolated CD4 and CD8 T cells from mice treated with the combination of intratumoral CpG and ibrutinib, and co-transplanted them with fresh tumor cells into adoptive naïve hosts (Figure 4A). Outgrowth of tumor was monitored. As expected, T cells from nontreated, ibrutinib alone treated, and the CpG alone treated groups failed to prevent tumor outgrowth (Figure 4B-G). The tumor outgrowth was prevented only when CD4 and CD8 from the combination treated mice were combined (Figure 4H); neither CD4 nor CD8 cells alone were able to prevent the growth (Figure 4I-J). The specificity of the immune memory was further demonstrated since the splenocytes from the mice treated with ibrutinib and CpG did not protect the co-transplant of an irrelevant cell line, BL-3750 (see supplemental Figure 1, available on the Blood Web site).
Transferred T cells from ibrutinib-CpG–treated mice prevent tumor growth in adoptive hosts. (A) Negatively selected CD3, CD8, or CD4 T-cell populations were mixed with H11 tumor cells (100:1 ratio) and co-injected into naïve C57BL/6 mice. (B-D) CD3, CD4, or CD8 T cells, respectively, from naïve mice. (E-G) CD3 T cells from tumor-bearing animals, untreated, ibrutinib-treated, or CpG-treated, respectively. (H-J) CD3, CD4, or CD8 T cells, respectively, from mice treated with the combination of ibrutinib and CpG. (n = 5 mice per group; this experiment was repeated twice with similar results.)
Transferred T cells from ibrutinib-CpG–treated mice prevent tumor growth in adoptive hosts. (A) Negatively selected CD3, CD8, or CD4 T-cell populations were mixed with H11 tumor cells (100:1 ratio) and co-injected into naïve C57BL/6 mice. (B-D) CD3, CD4, or CD8 T cells, respectively, from naïve mice. (E-G) CD3 T cells from tumor-bearing animals, untreated, ibrutinib-treated, or CpG-treated, respectively. (H-J) CD3, CD4, or CD8 T cells, respectively, from mice treated with the combination of ibrutinib and CpG. (n = 5 mice per group; this experiment was repeated twice with similar results.)
Taken together, these results demonstrate the direct role of both CD4 and CD8 T cells in the antitumor effect by ibrutinib and intratumoral CpG.
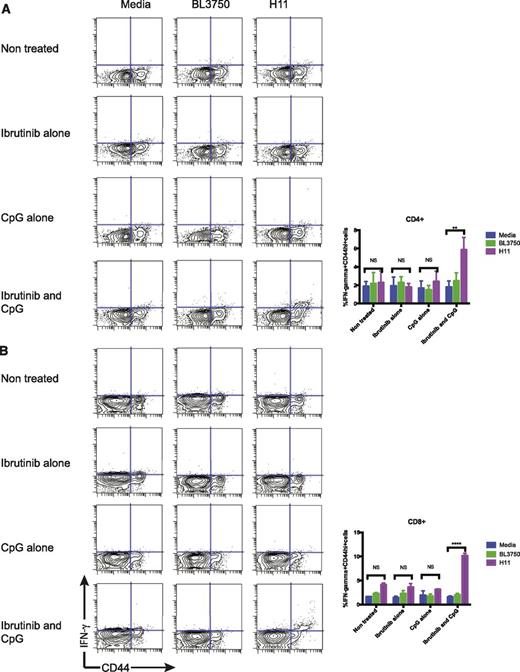
To further identify the tumor reactive T cells from the treated mice, we collected splenocytes following the treatment, and cultured them with irradiated H11 tumor cells or with various positive or negative control stimuli and measured the induction of intracellular IFN-γ in the T cells. Only the T cells from mice that were treated with both CpG and ibrutinib responded to the H11 tumor. Approximately 6% of CD4 T cells and 11% of CD8 T cells of total lymphocytes produced IFN-γ, in response to coculture with H11 tumor cells (Figure 5A-B). The tumor-specific, IFN-γ–producing, CD8 and CD4 T-cell response occurred among the memory CD44hi T-cell subset. This response was tumor-specific, since coculture with BL3750, an irrelevant C57/BL6 B-cell tumor, failed to induce IFN-γ expression.
Antitumor T-cell responses. Intracellular IFN-γ production by (A) CD4+ cells and (B) CD8+ cells. On day 4 posttreatment, splenocytes from the treated mice were cocultured with either media, irradiated BL3750 or H11 cells for 24 hours, and was in the presence of monensin for the last 5 to 6 hours. Intracellular IFN-γ were assayed by flow cytometry and results were gated on CD3+ cells; indicated are the proportion of IFN-γ+ cells as a percentage of total lymphocytes.
Antitumor T-cell responses. Intracellular IFN-γ production by (A) CD4+ cells and (B) CD8+ cells. On day 4 posttreatment, splenocytes from the treated mice were cocultured with either media, irradiated BL3750 or H11 cells for 24 hours, and was in the presence of monensin for the last 5 to 6 hours. Intracellular IFN-γ were assayed by flow cytometry and results were gated on CD3+ cells; indicated are the proportion of IFN-γ+ cells as a percentage of total lymphocytes.
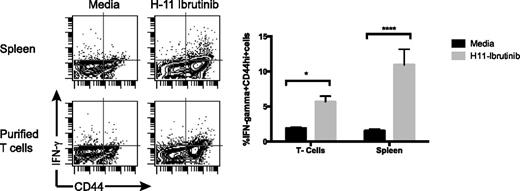
We wanted to test the hypothesis that ibrutinib-exposed tumor cells can release antigens to be presented to T cells. We cocultured splenocytes or purified T cells with H11 cells that were pretreated with ibrutinib and measured the induction of intracellular IFN-γ in the responding T cells. Immune T cells did respond to H11 tumor cells exposed to ibrutinib and this response was enhanced in the presence of APCs (Figure 6).
Ibrutinib-exposed tumor cells present their antigens to T cells. H11 tumor cells were incubated with ibrutinib (1 µM) for 24 hours and then cocultured with splenocytes or purified splenic T cells from mice that had been treated with the ibrutinib/CpG combination. IFN-γ production by CD3+/CD8+ T cells was measured by flow cytometry after 24 hours. The proportion of IFN-γ+ cells as a percentage of lymphocytes was calculated and shown as dot plots and as bar graphs. This response was higher when splenic APCs were present.
Ibrutinib-exposed tumor cells present their antigens to T cells. H11 tumor cells were incubated with ibrutinib (1 µM) for 24 hours and then cocultured with splenocytes or purified splenic T cells from mice that had been treated with the ibrutinib/CpG combination. IFN-γ production by CD3+/CD8+ T cells was measured by flow cytometry after 24 hours. The proportion of IFN-γ+ cells as a percentage of lymphocytes was calculated and shown as dot plots and as bar graphs. This response was higher when splenic APCs were present.
Discussion
We have demonstrated a powerful combination of ibrutinib and intratumoral injection of CpG for the induction of a therapeutic antitumor T-cell immune response. This T-cell response was long lasting, tumor specific, and composed of both CD4 and CD8 T cells. Neither the CpG nor the ibrutinib treatment alone, could induce this T-cell response. This T-cell response was detectable in vitro only in the mice receiving the combination of the two agents. Moreover, the T cells from the mice that were treated with the combination were able to prevent the outgrowth of the tumor in newly recipient mice when T cells from mice treated with either agent alone were not able to do so.
Our original concept was that ibrutinib would kill a tumor by targeting BTK, a critical signaling pathway in normal and malignant B-lineage cells. As we demonstrated in vitro, antigens released by the dying tumor, would be taken up by APCs in the tumor microenvironment and then, locally injected CpG would enhance their ability to present tumor antigens to T cells. The antitumor T cells could then travel throughout the body, attacking tumors even in noninjected sites. Consistent with these data, the combined therapeutic effect of ibrutinib and CpG injection was dependent on the tumor being sensitive to ibrutinib. From this, we conclude that direct cytotoxicity in vitro predicts the ability of the drug plus co-injected CpG to activate T-cell immunity. This is in spite of the observation that the drug alone does not dramatically delay tumor growth in vivo. This suggests that cell death is limited but sufficient in vivo to release antigens to activate immunity. In this view, ibrutinib, by killing some of the tumor cells was sufficient to substitute the cytotoxic chemotherapy and radiotherapy that we had used in the past to release tumor antigens and to initiate this chain of events.4
We demonstrated that the in vitro T-cell response was augmented by the presence of APCs, suggesting that antigens from an ibrutinib-treated tumor could be presented to T cells. However, a formal demonstration of crosspresentation would require the use of transport-associated protein-deficient APC and tumor cells of a given haplotype, inducing T-cell responses in a different haplotype.
But it is possible that other mechanisms are involved in the in vivo therapeutic effect. For instance, ibrutinib could have had a dual role to play in this antitumor response. In addition to its direct tumor killing function, it could also have affected the character and potency of the T-cell immune response, based on its known inhibition of ITK and the consequent shift in balance between Th1 and Th2 cells.8 In this regard, ibrutinib could be acting both as a tumor cytotoxic agent and as an immunologic adjuvant. Consistent with this view is the fact that ibrutinib, at the dose of 6 mg/kg that we used in these experiments, fully occupied BTK but by itself had only a minor therapeutic effect on these tumors in vivo, despite their sensitivity to the drug in vitro. To confirm that ibrutinib irreversibly binds both BTK and ITK, we conducted a probe assay on mouse spleen and thymus. The data revealed that under the conditions of our experiment, approximately 75% of ITK was covalently bound by ibrutinib.21
It has been reported that signaling via TLRs 8 and 9 requires BTK,22 and therefore, inhibition of BTK signaling by ibrutinib might have lead to a reduction in CpG-induced responses. Such interference could be addressed by sequential treatment with the two agents. However, in our in vivo experiments, we did not observe loss of effect of CpG when combined with ibrutinib. Moreover, it has also been reported that btk−/− DCs exhibit a more mature phenotype and stimulate T cells better than do wild-type DCs,23 suggesting that ibrutinib treatment could even enhance CpG-induced DC potency.
CpG itself induced potent killing of tumor cells at the injected site due to its activation of NK cells and macrophages. But no subsequent systemic immune response occurred with local CpG injection alone. The addition of ibrutinib made the difference between local and systemic antitumor effects mediated by CD4 and CD8 T cells. Ibrutinib has now been approved for the treatment of both CLL24 and mantle cell lymphoma,25 and it is therefore available for clinical testing in other tumor types as a single agent and in combination with a variety of other antitumor therapies.
The antitumor response by the combination treatment was very rapid, suggesting that the tumor is immunogenic and that preactivated immune cells existed in the host at the time that therapy began. These preexisting T cells were likely against retroviral antigens or overexpressed/modified cellular antigens, known to be present and immunogenic cell lines. The combination of CpG and ibrutinib boosted those T cells and enabled the rapid response. The tumor-specific, IFN-γ–producing, T-cell response is primarily among memory CD44hi T cells, consistent with the immune protection against mice rechallenge at a later time point.
T-cell unresponsiveness to tumor antigens is a common phenomenon and it is caused by a variety of factors produced by tumor cells and by the tumor microenvironment. To overcome tumor tolerance, several immunotherapy approaches have been employed, the most impressive of which has been the use of antibodies against targets such as CTLA4 and PD1 that mediate the negative influences of tumor cells and T-regulatory cells on T-effector cells. We have explored local intratumoral injection of CpG and such checkpoint antibodies, and found impressive therapeutic potential in preclinical animal models and now in human clinical trials as well.26
The results described in this study are based on syngeneic transplantable mouse tumor models that have their limitations but are nevertheless, useful for studying interactions between various therapies on the host immune system. Results in similar animal models were recently translated by Kolstad et al who demonstrated systemic antitumor T-cell responses in follicular lymphoma patients who were given local radiotherapy, intranodal injections of rituximab, immature DCs, and granulocyte macrophage-colony stimulating factor at the same time.27
An alternative would have been to use a genetically modified mouse model with spontaneous lymphoma induction. But spontaneous models also have issues of relevance to naturally occurring cancer because they are often based on forced or overexpression of driver oncogenes that override host-tumor interactions. And they may not contain the types of passenger mutations that are now thought to be important targets of T cells. Based on the result from the experiments presented here, there is strong rationale for adding ibrutinib to ongoing clinical trials of locally injected CpG in lymphoma (NCT00880581 and NCT01745354).
Presented in abstract form at the 1st American Society of Hematology meeting on lymphoma biology, Colorado Springs, CO, August 10, 2014.
Presented in abstract form at the 56th annual meeting of the American Society of Hematology, San Francisco, CA, December 6, 2014.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by a grant from the National Institutes of Health, National Cancer Institute (CA34233) and the William Lawrence and Blanche Hughes Foundation.
Authorship
Contribution: I.S.-B., L.B., D.K.C., and H.E.K. performed experiments, analyzed results, and created the figures; and R.L. and I.S.-B. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ronald Levy, Division of Oncology, Stanford University, 269 Campus Dr, CCSR 1105, Stanford, CA 94305; e-mail: levy@stanford.edu.