Key Points
IAIP and its anionic carbohydrate moieties bind and inhibit the damaging effects of histones both in vitro and in vivo.
Cell-surface–associated negatively charged glycocalyx and matrix-associated glycosminoglycans protect against histone-induced cytotoxicity.
Abstract
Extracellular histones are mediators of tissue injury and organ dysfunction; therefore they constitute potential therapeutic targets in sepsis, inflammation, and thrombosis. Histone cytotoxicity in vitro decreases in the presence of plasma. Here, we demonstrate that plasma inter-α inhibitor protein (IAIP) neutralizes the cytotoxic effects of histones and decreases histone-induced platelet aggregation. These effects are mediated through the negatively charged glycosaminoglycans (GAGs) chondroitin sulfate and high-molecular-weight hyaluronan (HMW-HA) associated with IAIP. Cell surface anionic glycosaminoglycans heparan sulfate and HA protect the cells against histone-mediated damage in vitro. Surface plasmon resonance showed that both IAIP and HMW-HA directly bind to recombinant histone H4. In vivo neutralization of histones with IAIP and HMW-HA prevented histone-induced thrombocytopenia, bleeding, and lung microvascular thrombosis, decreased neutrophil activation, and averted histone-induced production of inflammatory cytokines and chemokines. IAIP and HMW-HA colocalized with histones in necrotic tissues and areas that displayed neutrophil extracellular traps. Increasing amounts of IAIP-histone complexes detected in the plasma of septic baboons correlated with increase in histones and/or nucleosomes and consumption of plasma IAIP. Our data suggest that IAIP, chondroitin sulfate, and HMW-HA are potential therapeutic agents to protect against histone-induced cytotoxicity, coagulopathy, systemic inflammation, and organ damage during inflammatory conditions such as sepsis and trauma.
Introduction
Extracellular histones released passively from dead cells or actively as components of neutrophil extracellular traps (NETs) elicit major cytotoxic, thrombotic, and inflammatory effects (reviewed in Martinod and Wagner1 ). Levels of histone-DNA complexes (nucleosomes) are elevated in patients with sepsis, trauma, stroke, and coronary artery disease.2-5 Infusion of calf thymus histones into mice results in severe thrombocytopenia, increased bleeding time,6 and pathologic responses similar to sepsis, including migration of leukocytes into tissues, diffuse microvascular thrombosis, and fibrin and platelet deposition in the lung alveoli, which lead to ischemia reperfusion, multiple organ injury, and death.7 Extracellular histones are significant mediators and are therefore important therapeutic targets in inflammation and thrombosis.7-9
In vitro, histone cytotoxicity is significantly decreased in the presence of plasma or serum.10-12 Several plasma molecules with antihistone properties are known, including activated protein C (APC),7 albumin,13 heparins,6,14 and pentraxins PTX3,15 SAP,16 and C-reactive protein (CRP).17 Addition of histones to serum and plasma induces protein aggregation. Pemberton et al18 characterized the histone-bound proteins by shotgun proteomics and identified a total of 36 protein subunits, including complement components, coagulation factors, protease inhibitors, and apolipoproteins. Inter-α inhibitor protein (IAIP) was prominent among the histone-precipitated proteins. IAIP is an endogenous plasma serine protease inhibitor composed of 1 light chain and 2 heavy chains (HCs).19 The light chain, called bikunin, is a broad-specificity Kunitz-type proteinase inhibitor that carries a chondroitin sulfate (CS) moiety covalently linked to HC.20,21 During inflammation, IAIP interacts with tumor necrosis factor (TNF)-stimulated gene 6 protein (TSG-6), which supports trans-esterification of HC to hyaluronan (HA).22 Despite being relatively abundant in plasma (0.6 to 1.2 g/L), IAIP is considered a minor protease inhibitor under physiologic conditions.23 Alternative functions of IAIP have been sought and investigated. Here, we demonstrate that IAIP binds to extracellular histones and neutralizes their toxic effects both in vitro and in vivo, most likely through mechanism(s) involving the carbohydrate moieties associated with IAIP.
Methods
Materials
We used calf thymus histone type II (CTH II; Sigma-Aldrich, St. Louis, MO), as well as recombinant histones r-H2, r-H2A, r-H2B, r-H3, and r-H4 (New England Biolabs, Ipswich, MA). Bovine thrombin; human fibrinogen; HA sodium salt from bovine vitreous humor; Select-HA Hyaluronan 150, 500, and 1000 kDa; RPMI-1640 medium with glutamine; Polybrene; and chondroitinase ABC from Proteus vulgaris were purchased from Sigma-Aldrich. IAIP was isolated from fresh frozen human plasma by cryoprecipitation, solid-phase extraction, and ion-exchange chromatography.24 Bikunin (Urinary Trypsin Inhibitor-Ulinastatin Human) was from ProSpec (East Brunswick, NJ).
Cell culture
HL-60 cells (American Type Culture Collection [ATCC], Manassas, VA) were cultured in RPMI with 10% heat-inactivated fetal bovine serum (FBS) (HyClone, Thermo-Fisher Scientific, Waltham, MA), 100 U/mL penicillin, and 100 µg/mL streptomycin at 37°C in humidified 5% CO2 atmosphere. Chinese hamster ovary K1 (CHO-K1) and CHO-A745 cells (ATCC) were grown in Ham’s F-12K medium (ATCC) containing 10% FBS and antibiotics. Endothelial cell line EA.hy926 was grown in Dulbecco’s modified Eagle medium containing 10% FBS, 4.5 g/L glucose, l-glutamine, and antibiotics. Experiments were performed at 95% confluence.
Histone-induced cytotoxicity
HL-60 cells were incubated with 50 μg/mL CTH for 30 minutes at 37°C. In some experiments, histones were preincubated with IAIP (25-100 μg/mL), bikunin (100-500 μg/mL), or high-molecular-weight HA (HMW-HA; 100-500 μg/mL) for 15 minutes. Cytotoxicity was measured by propidium iodide (PI) staining through flow cytometry. To test for the role of HA negative charge, IAIP (100 µg/mL) and HMW-HA (500 µg/mL) were incubated with the cationic polymer Polybrene (0.5 mg/mL) for 15 minutes before adding over the cells. HL-60 cells were also incubated with IAIP that was pretreated with chondroitinase ABC (0.05 IU/mL) for 18 hours at 37°C to remove its CS moiety.
EA.hy926 cells were incubated with histones (50 µg/mL, 30 minutes), either alone or after preincubation with 100 μg/mL IAIP, 500 μg/mL HMW-HA, or 500 μg/mL bikunin. PI was added to each well (10 μg/mL) and incubated for 10 minutes. Fluorescence was recorded at excitation and emission of 540 nm and 620 nm, respectively. This measurement reflects the histone-induced membrane permeability. Triton-X100 (2.5 g/L final concentration) was added for 30 minutes to permeabilize all cells. Fluorescence intensity was remeasured to quantify the total number of cells. The percentage of histone-induced cell death was reported as the ratio between the two measurements multiplied by 100.
CHO-K1 and CHO-A745 cells were incubated with 50 µg/mL histone for 4 hours and membrane permeability to PI was measured by fluorimetry, as above.
Isolation of human platelets
Blood was collected by venipuncture with a 16-gauge needle from drug-free healthy volunteers into acid-citrate-dextrose (6:1 ratio). Institutional review board approval was received from the Oklahoma Medical Research Foundation. Platelet-rich plasma was prepared by centrifugation at 180g for 10 minutes at room temperature. Platelet-rich plasma was then centrifuged at 400g, and platelets were suspended in N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid-Tyrode’s buffer (140 mM NaCl, 2.7 mM KCl, 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 0.2 mM Na2HPO4, 12 mM NaHCO3, 5.5 mM glucose, 1 mM MgCl2) at pH 7.4.
Platelet aggregometry
Platelet aggregation was determined in washed platelets in the presence of 4 mM CaCl2 and human fibrinogen (200 µg/mL) using a PAP-8E optical aggregometer (Bio/Data Corporation, Horsham, PA). Platelet aggregation was induced with r-H4, preincubated or not with IAIP, HMW-HA, or bikunin at the concentrations indicated in the figure legends. Thrombin (0.5 U/mL) and vehicle served as positive and negative controls, respectively. In some experiments, r-H4–induced platelet aggregation was determined in washed platelets suspended in Tyrode’s buffer supplemented with 20% control plasma or IAIP-depleted plasma in the presence or absence of IAIP (100 µg/mL) or CRP (250 µg/mL).
In vivo histone challenge in mice
We used 6- to 10-week-old male C57BL/6 mice from The Jackson Laboratory (Bar Harbor, ME) according to an animal protocol approved by the Institutional Animal Care and Use Committee of the Oklahoma Medical Research Foundation.
CTH (50 mg/kg) or histones preincubated with IAIP (50 mg/kg) or HMW-HA (90 mg/kg) were administered by retro-orbital injection. In some experiments, HMW-HA was preadministered 15 minutes before histones. Blood was collected on 5 mM EDTA from the submandibular vein before challenge and by cardiac puncture 3 hours after challenge. Blood cell counts were determined by using a Hemavet HV950S Hematology System (Drew Scientific, Waterbury, CT). Blood was centrifuged for 10 minutes at 3000g, and plasma was stored at −80°C for cytokines and myeloperoxidase (MPO) analysis.
Bleeding time measurement
Tail bleeding time was determined 30 minutes after challenge by removing 1 cm of the distal mouse tail and immersing the tail in phosphate-buffered saline at 37°C.25 A complete cessation of bleeding for >120 seconds was defined as the bleeding time. Bleeding times >600 seconds were stopped by ligation of the tail.
Biochemical analysis
MPO activity was measured with a Fluoro MPO activity kit from Cell Technology, Inc. (Mountain View, CA).
Multiplex enzyme-linked immunosorbent assay for cytokines
Mouse plasma cytokines were measured 3 hours after challenge by using a 6-plex xMAP cytokine/chemokine magnetic bead panel (interleukin 1β [IL-1β], IL-6, IL-10, keratinocyte chemoattractant (KC), monocyte chemoattractant protein-1 [MCP-1], and TNF-α) from EMD Millipore (Billerica, MA) using a Bio-Plex 200 suspension array system (BioRad, Hercules, CA).
Statistics
All results are expressed as mean ± standard error of the mean (SEM). Comparison between 2 independent groups was made by Student t test. Multiple group analysis was performed by analysis of variance followed by Bonferroni’s multiple comparison tests using Prism (GraphPad Software, San Diego, CA). Differences were considered significant at P < .05. More information about methods can be found in the supplemental Data (available online on the Blood Web site).
Results
IAIP and HWM-HA, but not bikunin, neutralize the cytotoxic effects of extracellular histones
Our group showed that histones are cytotoxic to endothelial cells in culture, as measured by PI staining.7 Since IAIP in plasma is decreased during sepsis26 and is also one of the plasma proteins that are precipitated by histones,18 we tested whether IAIP could abrogate the cytotoxicity of histones. Incubation of HL-60 cells with CTH resulted in rapid PI uptake, indicating loss of plasma membrane integrity. IAIP dramatically reduced histone cytotoxicity in a concentration-dependent manner (Figure 1A).
IAIP and HMW-HA, but not bikunin, prevent histone-induced cytotoxicity. Cell viability was measured by flow cytometry using PI staining in HL-60 cells incubated with CTH (50 µg/mL) at 37°C for 30 minutes in the presence or absence of (A) IAIP 25 to 100 µg/mL, (B) HA 250 to 500 µg/mL, and (C) bikunin 100 to 500 µg/mL. (D) Cell viability measured as above in EA.hy926 endothelial cell line in the presence or absence of IAIP 100 µg/mL, HA 500 µg/mL, or bikunin 500 µg/mL. (E) Effect of chondroitinase ABC (C-ABC) on cell protective effect of IAIP. HL-60 cells were incubated with histones with or without IAIP pretreated or not with C-ABC (0.05 IU/mg, 18 hours at 37°C) before analysis with flow cytometry. C-ABC alone did not affect cell viability. (F) Polybrene (PB) abolishes the cytoprotective effects of IAIP and HA. HL-60 cells were incubated with histones with or without IAIP or HA pretreated or not with Polybrene (0.5 mg/mL). Polybrene alone does not affect histone cytotoxicity and has no direct effect on cell viability. Data are presented as mean ± standard error of the mean (SEM) of 3 independent experiments using one-way analysis of variance (ANOVA) with Bonferroni’s multicomparison test. ****P < .0001, ***P < .001, and **P < .01 compared with the histone challenge group. NS, not significant.
IAIP and HMW-HA, but not bikunin, prevent histone-induced cytotoxicity. Cell viability was measured by flow cytometry using PI staining in HL-60 cells incubated with CTH (50 µg/mL) at 37°C for 30 minutes in the presence or absence of (A) IAIP 25 to 100 µg/mL, (B) HA 250 to 500 µg/mL, and (C) bikunin 100 to 500 µg/mL. (D) Cell viability measured as above in EA.hy926 endothelial cell line in the presence or absence of IAIP 100 µg/mL, HA 500 µg/mL, or bikunin 500 µg/mL. (E) Effect of chondroitinase ABC (C-ABC) on cell protective effect of IAIP. HL-60 cells were incubated with histones with or without IAIP pretreated or not with C-ABC (0.05 IU/mg, 18 hours at 37°C) before analysis with flow cytometry. C-ABC alone did not affect cell viability. (F) Polybrene (PB) abolishes the cytoprotective effects of IAIP and HA. HL-60 cells were incubated with histones with or without IAIP or HA pretreated or not with Polybrene (0.5 mg/mL). Polybrene alone does not affect histone cytotoxicity and has no direct effect on cell viability. Data are presented as mean ± standard error of the mean (SEM) of 3 independent experiments using one-way analysis of variance (ANOVA) with Bonferroni’s multicomparison test. ****P < .0001, ***P < .001, and **P < .01 compared with the histone challenge group. NS, not significant.
Histones are polycationic proteins with high affinity for glycosaminoglycans (GAGs).6 To determine which one of the moieties associated with IAIP, the GAGs or the bikunin polypeptide, mediate the cytoprotective effect, we incubated HL-60 cells with CTH in the presence or absence of HMW-HA or bikunin at different concentrations. We found that HMW-HA significantly decreased histone-induced cytotoxicity (Figure 1B), but bikunin had no effect (Figure 1C).
Similarly, we observed that IAIP and HA, but not bikunin, decreased PI uptake in endothelial cells (Figure 1D). Pretreatment of IAIP with chondroitinase ABC, an enzyme that degrades both CS and HA moieties to disaccharides, abolished the protective effects of IAIP (Figure 1E), suggesting that these are mediated by the carbohydrates associated with IAIP. To test whether the neutralization of the electric charge of histones by anionic carbohydrates is responsible for the observed cytoprotection, IAIP and HA were preincubated with Polybrene, a cationic polymer that interacts with highly charged sugars.27 Polybrene incubation abolished the protective effects of IAIP and HA without having direct effects on histones or cells by itself (Figure 1F). These data suggest that CS and HA moieties of the IAIP complex protect against histone-induced cell death.
One major mechanism of histone-induced cytotoxicity in EA.hy926 cells is through binding phospholipids, disrupting cell membranes, and causing calcium influx.28 We tested whether histones likewise induce calcium influx in HL-60 cells by using a calcium-sensitive fluorophore, Fluo-3. Like in EA.hy926 cells, histones also increased calcium influx in HL-60 cells. Preincubating histones with IAIP or HMW-HA, but not bikunin, significantly reduced the histone-triggered calcium influx (supplemental Figure 1). These results indicate that both IAIP and HMW-HA prevent histone-induced cytotoxicity, possibly by reducing the subsequent calcium influx, whereas bikunin has no effect.
Cell glycocalyx counteracts histone-induced cytotoxicity
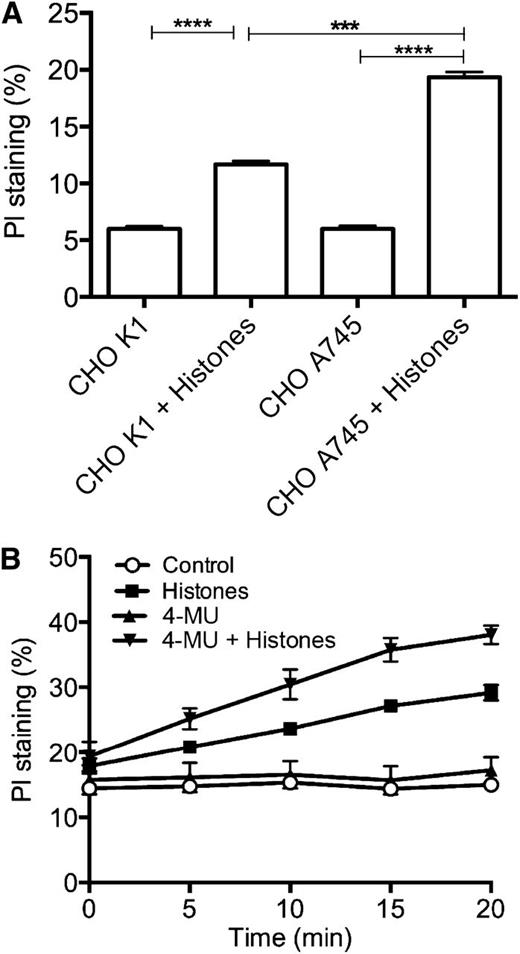
Next, we investigated whether the cell surface anionic GAGs protect against histone-induced cytotoxicity. We compared the effects of histones on CHO-K1 cells that express heparan sulfate vs the mutant CHO-A745 cells that are deficient in this GAG.29 We observed that the mutant cells are significantly more sensitive to histone challenge (Figure 2A), suggesting that cell surface glycocalyx plays an important protective role against histones.
Cell surface glycocalyx protects against histone-induced cell death. (A) CHO-K1 and CHO-A745 cells were incubated with or without histones (50 µg/mL for 4 hours at 37°C), and membrane permeability to PI was measured by fluorimetry. (B) EA.hy926 cells grown in the presence or absence of 4-MU (100 µM for 24 hours at 37°C) were incubated with or without histones (50 µg/mL), and membrane permeability to PI was measured by fluorimetry. Data are presented as mean ± SEM of 3 independent experiments using one-way ANOVA with Bonferroni’s multicomparison test. ****P < .0001, ***P < .001.
Cell surface glycocalyx protects against histone-induced cell death. (A) CHO-K1 and CHO-A745 cells were incubated with or without histones (50 µg/mL for 4 hours at 37°C), and membrane permeability to PI was measured by fluorimetry. (B) EA.hy926 cells grown in the presence or absence of 4-MU (100 µM for 24 hours at 37°C) were incubated with or without histones (50 µg/mL), and membrane permeability to PI was measured by fluorimetry. Data are presented as mean ± SEM of 3 independent experiments using one-way ANOVA with Bonferroni’s multicomparison test. ****P < .0001, ***P < .001.
We verified these findings by incubating EA.hy926 cells with 4-methylumbelliferone (4-MU), which inhibits HA synthesis,30 and testing the effects of histones in the PI membrane permeability assay. 4-MU–treated cells were more sensitive to histones than nontreated controls (Figure 2B), further supporting the role of GAGs in maintaining the integrity of the plasma membrane.
IAIP and HA reduce histone-induced platelet aggregation, prothrombinase activity, and blood clotting time
Extracellular histones are prothrombotic.2,6,8,9 In vitro, r-H4 induces aggregation of platelets as effectively as thrombin.6,13 To assess the effect of IAIP on histone-induced platelet aggregation, we incubated washed platelets with r-H4 (20 µg/mL) in the presence or absence of IAIP. As shown in Figure 3A, pretreatment of r-H4 with IAIP reduced platelet aggregation to control levels, and the effect was concentration dependent. To determine the relative contribution of IAIP to histone inhibition compared with other plasma proteins, r-H4 was incubated with washed platelets suspended in 20% integral, cryo-poor (CP) or IAIP depleted CP plasma with or without supplementation with exogenous IAIP (100 µg/mL), albumin (5 mg/mL), or CRP (250 µg/mL). We observed that integral or CP, but not IAIP CP-depleted plasma, inhibits r-H4–induced platelet aggregation (Figure 3B). Addition of IAIP, but not CRP or albumin, recovers r-H4 inhibitory properties of plasma, suggesting that IAIP is the main histone inhibitor in the plasma. This conclusion was supported by competitive assays in which histone-IAIP interaction was competed with increasing amounts of albumin or CRP, both known to interact with histones.13,16,17 CRP did not inhibit histone-IAIP, even when used at the high concentration typical of inflammatory conditions, whereas albumin showed 20% inhibition only at 50 mg/mL (supplemental Figure 2).
Effect of IAIP, HMW-HA, and bikunin on histone-induced platelet aggregation. (A) Washed platelets were incubated with r-H4 (20 µg/mL) with or without IAIP (50 to 200 µg/mL) or HMW-HA (100 to 500 µg/mL) before aggregometry. Thrombin (0.5 U/mL)–induced aggregation was measured as positive control. H4-induced platelet aggregation was decreased by IAIP in a concentration-dependent manner when platelets were suspended in plasma. (B) Washed platelets were incubated with integral (total), cryo-poor (CP) or IAIP-depleted (depl.) CP plasma containing or not 20 µg/mL r-H4. In some experiments, IAIP-depleted CP plasma was supplemented with IAIP (100 µg/mL), CRP (250 µg/mL), or albumin (Alb; 5 mg/mL). H4-induced aggregation was dependent on the amount of IAIP but not on CRP or albumin in plasma. (C-D) Washed platelets were incubated with r-H4 with or without increasing concentrations of (C) HMW-HA or (D) bikunin. (C) HMW-HA had a dose-dependent inhibitory effect on histone-induced platelet aggregation, whereas (D) preincubation of H4 with bikunin did not affect platelet aggregation. Data are presented as mean ± SEM of 3 independent experiments using one-way ANOVA with Bonferroni’s multi-comparison test. ****P < .0001, ***P < .001, and **P < .01 compared with the H4 challenge group.
Effect of IAIP, HMW-HA, and bikunin on histone-induced platelet aggregation. (A) Washed platelets were incubated with r-H4 (20 µg/mL) with or without IAIP (50 to 200 µg/mL) or HMW-HA (100 to 500 µg/mL) before aggregometry. Thrombin (0.5 U/mL)–induced aggregation was measured as positive control. H4-induced platelet aggregation was decreased by IAIP in a concentration-dependent manner when platelets were suspended in plasma. (B) Washed platelets were incubated with integral (total), cryo-poor (CP) or IAIP-depleted (depl.) CP plasma containing or not 20 µg/mL r-H4. In some experiments, IAIP-depleted CP plasma was supplemented with IAIP (100 µg/mL), CRP (250 µg/mL), or albumin (Alb; 5 mg/mL). H4-induced aggregation was dependent on the amount of IAIP but not on CRP or albumin in plasma. (C-D) Washed platelets were incubated with r-H4 with or without increasing concentrations of (C) HMW-HA or (D) bikunin. (C) HMW-HA had a dose-dependent inhibitory effect on histone-induced platelet aggregation, whereas (D) preincubation of H4 with bikunin did not affect platelet aggregation. Data are presented as mean ± SEM of 3 independent experiments using one-way ANOVA with Bonferroni’s multi-comparison test. ****P < .0001, ***P < .001, and **P < .01 compared with the H4 challenge group.
Next, we pretreated r-H4 with HMW-HA or bikunin. Similar to IAIP, HMW-HA inhibited histone-induced platelet aggregation in a concentration-dependent manner (Figure 3C). Increasing levels of bikunin had no inhibitory effect on histone-induced platelet aggregation (Figure 3D).
Because platelet activation and aggregation are accompanied by the exposure of a phosphatidylserine-rich procoagulant surface, we tested the effects of histones with or without HA on platelet prothrombinase activity. Supplemental Figure 3 shows that platelet stimulation with r-H4 promoted prothrombinase activity, consistent with the exposure of anionic phospholipids. r-H4 induced thrombin generation similar to that of the positive control (ionomycin), but not to the same extent. Pretreatment of r-H4 with HA inhibited the H4-induced prothrombotic surface exposure in a dose-dependent manner. The prothrombinase activity of platelets treated with 50 to 100 µg/mL HA was indistinguishable from that of the nontreated platelet controls, and the values are given together as negative control. Overall, these data show that IAIP and HA protect against histone-induced damage of the plasma membrane integrity.
We also investigated the effect of IAIP and HA on histone-induced blood clotting by using a whole blood recalcification assay in the presence of diluted thromboplastin reagent (supplemental Figure 4). Histones (100 µg/mL) significantly accelerated blood clotting, which was abolished when histones were treated with IAIP or HA. IAIP or HA alone had no effect.
Effect of molecular weight of HA on histone-induced cytotoxicity and platelet aggregation
Differential biological effects are observed with high- and low-molecular-weight HA on cells and tissues.31 Here, we tested the possibility that the molecular weight of HA could influence its effects against histone toxicity in vitro. Although all forms of HA gave positive results, the effect was proportional with molecular weight, the HMW-HA of 1000 kDa showing more protection than the 500 kDa and 150 kDa forms of HA against histone-induced cytotoxicity (Figure 4A) and platelet aggregation (Figure 4B).
Effect of the molecular weight of HA on histone-induced cytotoxicity and platelet aggregation. (A) HL-60 cells were incubated with CTH (50 µg/mL) at 37°C for 30 minutes in the presence or absence of 100 µg/mL HA with a molecular weight of 150, 500, or 1000 kDa. Cell damage was measured by PI staining and flow cytometry. (B) Platelet aggregation was measured after 20 µg/mL H4 was added to washed platelets in the presence or absence of 100 µg/mL of HA at 150, 500, or 1000 kDa. Data are presented as mean ± SEM of 3 independent experiments using one-way ANOVA with Bonferroni’s multicomparison test. ***P < .001, **P < .01, *P < .05 compared with (A) histone or (B) H4 challenge groups.
Effect of the molecular weight of HA on histone-induced cytotoxicity and platelet aggregation. (A) HL-60 cells were incubated with CTH (50 µg/mL) at 37°C for 30 minutes in the presence or absence of 100 µg/mL HA with a molecular weight of 150, 500, or 1000 kDa. Cell damage was measured by PI staining and flow cytometry. (B) Platelet aggregation was measured after 20 µg/mL H4 was added to washed platelets in the presence or absence of 100 µg/mL of HA at 150, 500, or 1000 kDa. Data are presented as mean ± SEM of 3 independent experiments using one-way ANOVA with Bonferroni’s multicomparison test. ***P < .001, **P < .01, *P < .05 compared with (A) histone or (B) H4 challenge groups.
Histones bind with specificity and affinity to IAIP and HMW-HA
Formation of histone-IAIP complexes using increasing concentrations of the two binding partners showed that this interaction is concentration-dependent and saturable (supplemental Figure 5). To determine the differential binding between IAIP and the main histones, we performed pull-down assays in which microwells were coated with CTH or recombinant histones and incubated with IAIP (supplemental Figure 6). We observed that H1 and H4 pulled down larger amounts of IAIP than H2A, H2B, or H3. Since H3 and H4 are known to have cytotoxic and thrombogenic effects, we further investigated their interaction with IAIP and HA by using surface plasmon resonance. Biotinylated r-H4 and H3 were captured on streptavidin-coated sensor chips, and IAIP or HMW-HA was injected at increasing concentrations (supplemental Figure 7). For comparison, interaction of H4 and H3 with heparin, a known histone-binding GAG6 was determined. Surface plasmon resonance data revealed that H4 binds twofold to threefold more strongly to IAIP, HMW-HA, or heparin than H3 does. Our findings show that different histones have unequal binding to GAGs. Bikunin did not bind to H3 or H4 (data not shown). Altogether, our results suggest that direct binding of IAIP and HMW-HA to extracellular histones may prevent their toxic effects.
IAIP and HMW-HA protect against histone-induced thrombocytopenia and microvascular thrombosis
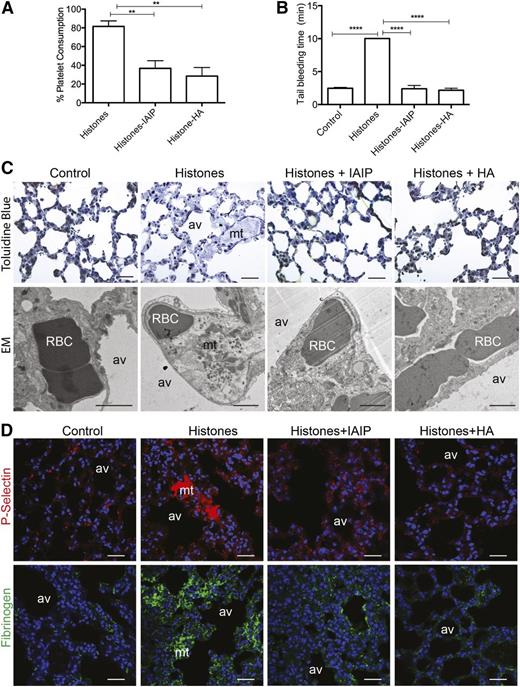
In vivo, histones strongly induce platelet activation and aggregation and promote coagulation and intravascular thrombosis.8,9,32 To determine whether IAIP and HMW-HA counteract histone-induced prothrombotic effects and platelet activation in vivo, we challenged mice with a sublethal dose of CTH (50 mg/kg). As expected, mice developed severe thrombocytopenia associated with prolonged bleeding time.6 Preincubation of histones with IAIP or HMW-HA significantly reduced histone-induced platelet consumption and bleeding time (Figure 5A-B). Similar effects were observed when HMW-HA was preinjected into mice 15 minutes before histones (supplemental Figure 8), demonstrating that HMW-HA efficiently inhibits histones in vivo. Electron microscopy and immunofluorescence staining for P-selectin of lung samples collected 3 hours after the challenge showed platelet-rich thrombi in mice injected with histones only, but not when histones were preincubated with IAIP or HMW-HA (Figure 5C-D). Similarly, lungs from mice injected with histones showed fibrin deposition, which was abrogated by preincubation with IAIP and HMW-HA (Figure 5D). These results suggest that IAIP and HMW-HA reduce histone-induced activation of coagulation.
IAIP and HMW-HA prevent histone-induced thrombocytopenia, prolonged bleeding time, microthrombosis, and fibrinogen accumulation. (A-B) Percentage of (A) platelet consumption and (B) tail bleeding time in mice injected intravenously with 50 mg/kg histones without (n = 6) or with preincubation with IAIP (50 mg/kg; n = 6) or HMW-HA (90 mg/kg; n = 6). Data are presented as mean ± SEM of 3 independent experiments using one-way ANOVA with Bonferroni’s multicomparison test. ****P < .0001 and **P < .01 compared with the histones challenge group. (C-D) Pathologic changes in mouse lungs 3 hours after intravenous injection of 50 mg/kg histones with or without preincubation with IAIP or HMW-HA. (C) Toluidine blue staining on semi-thin epon sections (upper row) and electron microscopy (EM; lower row), and (D) immunofluorescence staining for platelet marker P-selectin (upper row; red) and fibrinogen and/or fibrin (lower row; green) demonstrate the presence of platelet and fibrin-rich intravascular microthrombi (mt) in histone-only treated mice compared with controls and mice injected with histones pretreated with IAIP or HMW-HA. Magnification bars for (C): toluidine blue, 100 µm; EM, 1 µm; for (D): all bars are 100 µm. av, alveoli; RBC, red blood cells.
IAIP and HMW-HA prevent histone-induced thrombocytopenia, prolonged bleeding time, microthrombosis, and fibrinogen accumulation. (A-B) Percentage of (A) platelet consumption and (B) tail bleeding time in mice injected intravenously with 50 mg/kg histones without (n = 6) or with preincubation with IAIP (50 mg/kg; n = 6) or HMW-HA (90 mg/kg; n = 6). Data are presented as mean ± SEM of 3 independent experiments using one-way ANOVA with Bonferroni’s multicomparison test. ****P < .0001 and **P < .01 compared with the histones challenge group. (C-D) Pathologic changes in mouse lungs 3 hours after intravenous injection of 50 mg/kg histones with or without preincubation with IAIP or HMW-HA. (C) Toluidine blue staining on semi-thin epon sections (upper row) and electron microscopy (EM; lower row), and (D) immunofluorescence staining for platelet marker P-selectin (upper row; red) and fibrinogen and/or fibrin (lower row; green) demonstrate the presence of platelet and fibrin-rich intravascular microthrombi (mt) in histone-only treated mice compared with controls and mice injected with histones pretreated with IAIP or HMW-HA. Magnification bars for (C): toluidine blue, 100 µm; EM, 1 µm; for (D): all bars are 100 µm. av, alveoli; RBC, red blood cells.
Effect of IAIP and HMW-HA on histone-induced tissue damage and proinflammatory response
Histones can induce sterile inflammation, tissue injury, and death in mouse models,33 in part through production of proinflammatory cytokines.34 At 3 hours after challenge, mice injected with histones had increased plasma levels of proinflammatory cytokines (IL-1β, IL-6, TNF-α), chemokines (KC, MCP-1), and anti-inflammatory IL-10, whereas pretreatment of histones with IAIP or HA and preinjection of HMW-HA before histone challenge significantly decreased cytokine and chemokine production (Figure 6A). Immunohistochemistry with antineutrophil elastase antibody showed increased number of partially degranulated neutrophils in the lung of histone-challenged mice compared with controls (Figure 6B-C). Lung accumulation of neutrophils was paralleled by increased plasma MPO activity (Figure 6C). Preincubation of histones with IAIP and HMW-HA significantly decreased neutrophil infiltration.
In vivo effect of IAIP and HMW-HA in a mouse model of histone challenge. (A) Effect of IAIP and HMW-HA on histone-induced production of proinflammatory cytokines and chemokines. TNFα, IL-6, KC, IL-1β, monocyte chemoattractant protein-1 (MCP-1), and IL-10 levels in mouse plasma collected 3 hours after challenge with 50 mg/kg histones alone (n = 5) or preincubated with 50 mg/kg IAIP (n = 5) or 90 mg/kg HMW-HA (n = 5). Data are presented as mean ± SEM and analyzed with one-way ANOVA with Bonferroni’s multicomparison test. ****P < .0001, ***P < .001, **P < .01, and *P < .05 compared with the histones challenge group. (B) Immunostaining of neutrophil elastase in the lungs of mice injected with histones alone, histones incubated with IAIP or HMW-HA, and controls. Nuclei are shown in blue. Magnification bars: 100 µm. (C) Quantitation of neutrophils in the lungs; histogram data are shown as mean ± SEM for 10 microscopic fields collected from at least 3 different animals per condition. One-way ANOVA with Bonferroni’s multicomparison test was used; ***P < .001 compared with the histone challenge group. (D) Measurement of MPO activity in plasma from mice injected with histones only compared with controls and histones preincubated with IAIP or HMW-HA. Data are shown as mean ± SEM from 3 independent experiments using one-way ANOVA with Bonferroni’s multicomparison test. ***P < .001 and **P < .01 compared with the histone challenge group.
In vivo effect of IAIP and HMW-HA in a mouse model of histone challenge. (A) Effect of IAIP and HMW-HA on histone-induced production of proinflammatory cytokines and chemokines. TNFα, IL-6, KC, IL-1β, monocyte chemoattractant protein-1 (MCP-1), and IL-10 levels in mouse plasma collected 3 hours after challenge with 50 mg/kg histones alone (n = 5) or preincubated with 50 mg/kg IAIP (n = 5) or 90 mg/kg HMW-HA (n = 5). Data are presented as mean ± SEM and analyzed with one-way ANOVA with Bonferroni’s multicomparison test. ****P < .0001, ***P < .001, **P < .01, and *P < .05 compared with the histones challenge group. (B) Immunostaining of neutrophil elastase in the lungs of mice injected with histones alone, histones incubated with IAIP or HMW-HA, and controls. Nuclei are shown in blue. Magnification bars: 100 µm. (C) Quantitation of neutrophils in the lungs; histogram data are shown as mean ± SEM for 10 microscopic fields collected from at least 3 different animals per condition. One-way ANOVA with Bonferroni’s multicomparison test was used; ***P < .001 compared with the histone challenge group. (D) Measurement of MPO activity in plasma from mice injected with histones only compared with controls and histones preincubated with IAIP or HMW-HA. Data are shown as mean ± SEM from 3 independent experiments using one-way ANOVA with Bonferroni’s multicomparison test. ***P < .001 and **P < .01 compared with the histone challenge group.
IAIP and HMW-HA associates with histones and/or nucleosomes and NETs in blood and tissue samples from septic baboons
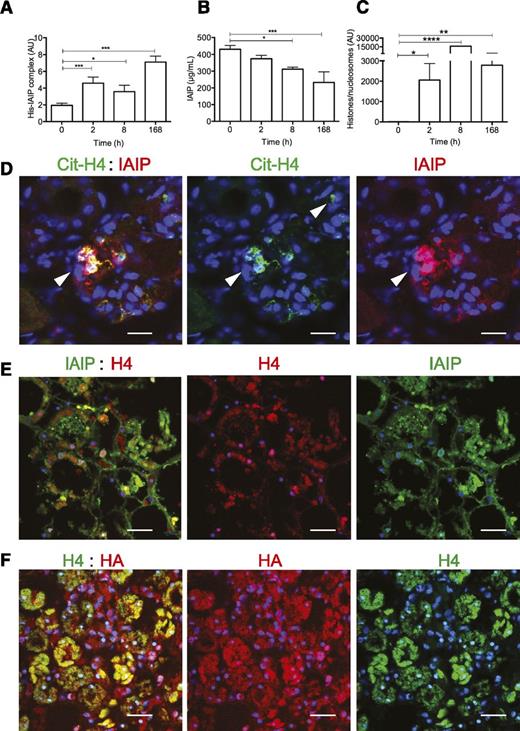
To test whether histone-IAIP complexes occur in vivo, we used a sandwich enzyme-linked immunosorbent assay that captured histones and detected the associated IAIP with an enzyme-labeled–specific antibody. We tested blood samples from historic experiments in which baboons were challenged with Escherichia coli.35 Histone-IAIP complexes correlated with the amounts of total IAIP and histones and/or nucleosomes detected in plasma. We observed a gradual increase in histone-IAIP complexes (Figure 7A) during sepsis progression, paralleled by IAIP consumption (Figure 7B) and increased histones and/or nucleosomes (Figure 7C) in plasma.
Detection of histone-HA and histone-IAIP complexes in plasma and tissues of baboons challenged with a lethal dose of E coli. (A) Histone-IAIP (His-IAIP) complexes, (B) total IAIP, and (C) histones and/or nucleosomes were detected in plasma of baboons challenged with E coli by intravenous infusion. T0 is the time of blood collection before E coli challenge. Data are shown as mean ± SEM of 3 independent experiments using one-way ANOVA with Bonferroni’s multicomparison test. ****P < .0001, ***P < .0001, **P < .01, and *P < .05 compared with the time of blood collection before E coli challenge (T0). (D) Immunohistochemical detection of IAIP (red) and cit-H4 with an antibody detecting citrulline at residue 3 (Cit-H4, green) in kidney sections from septic baboons shows colocalization of the 2 proteins (yellow) with neutrophil extracellular traps (arrows). Magnification bar: 50 µm. (E) Colocalization for IAIP (green) and H4 (red; overlap, yellow) in areas showing tubular necrosis in the kidney from a septic baboon. Magnification bars: 100 µm. (F) Colocalization of HA (red) with H4 (green) in areas with tubular necrosis of the kidney from a septic baboon. Magnification bars: 100 µm.
Detection of histone-HA and histone-IAIP complexes in plasma and tissues of baboons challenged with a lethal dose of E coli. (A) Histone-IAIP (His-IAIP) complexes, (B) total IAIP, and (C) histones and/or nucleosomes were detected in plasma of baboons challenged with E coli by intravenous infusion. T0 is the time of blood collection before E coli challenge. Data are shown as mean ± SEM of 3 independent experiments using one-way ANOVA with Bonferroni’s multicomparison test. ****P < .0001, ***P < .0001, **P < .01, and *P < .05 compared with the time of blood collection before E coli challenge (T0). (D) Immunohistochemical detection of IAIP (red) and cit-H4 with an antibody detecting citrulline at residue 3 (Cit-H4, green) in kidney sections from septic baboons shows colocalization of the 2 proteins (yellow) with neutrophil extracellular traps (arrows). Magnification bar: 50 µm. (E) Colocalization for IAIP (green) and H4 (red; overlap, yellow) in areas showing tubular necrosis in the kidney from a septic baboon. Magnification bars: 100 µm. (F) Colocalization of HA (red) with H4 (green) in areas with tubular necrosis of the kidney from a septic baboon. Magnification bars: 100 µm.
Next, we used immunocytochemistry to determine whether IAIP (Figure 7D-E) and HA (Figure 7F) are found in similar locations with native or citrullinated (cit) histone 4 (cit-H4) (in kidneys from baboons challenged with a lethal dose of E coli vs healthy controls). PAD4-mediated histone hypercitrullination is responsible for histone decondensation during NETs release.36 We observed a strong increase in colocalization of H4 or cit-H4 with IAIP and HA, particularly in areas showing tubular necrosis or structures that resemble NETs (Figure 7D-F), suggesting in situ association of extracellular histones and/or nucleosomes with IAIP and HA.
Discussion
Circulating extracellular histones, either actively secreted as NETs or derived from apoptotic cells, are major mediators and biomarkers of poor prognosis in sepsis,7 trauma,37 stroke,32 coronary artery disease,2 acute lung injury,28,38 and kidney injury.34 Direct intravenous injection of histones leads to sepsis-like microvascular thrombosis, organ dysfunction, and death at high doses.6,7 Histone-containing NETs promote platelet aggregation and thrombus formation.8,9 Both the proinflammatory and procoagulant effects of extracellular histones are linked to signaling via the Toll-like receptor 2 (TLR2) and TLR4.8,33,34 Histone inhibition with specific antibodies rescues lipopolysaccharide- or E coli–challenged mice,7 prevents endotoxin- and ischemia-reperfusion–induced acute kidney injury,34 prevents thrombocytopenia and platelet activation,6 and protects in ischemic stroke.32
In this study, we demonstrate that (1) negatively charged carbohydrate moieties of IAIP such as CS and HMW-HA bind to extracellular histones; (2) anionic GAGs HA and heparan sulfate in the cell surface glycocalyx protect against the cytotoxic effects of extracellular histones; and (3) the interaction between anionic carbohydrates and histones and/or nucleosomes occurs in vivo and may counterbalance the detrimental effects of histones in sepsis by reducing inflammation, microvascular thrombosis, and tissue injury. Our findings suggest that IAIP and HMW-HA could become novel therapies in inflammatory and prothrombotic diseases that involve neutrophil activation and cell death.
The physiologic function of IAIP remains elusive; however, several studies support a significant role in inflammation.39-41 In experimental models of sepsis, treatment with IAIP increased the survival rate and arrested progression of sepsis.42-44 IAIP levels decrease in patients with clinically proven sepsis26,45 and directly correlate with survival. Although the mechanism by which IAIP mediates its anti-inflammatory function is not known, TSG6-mediated HA transfer to the HC of IAIP could be responsible for these protective effects.22,39,46 Although IAIP treatment showed benefits in rodent models of sepsis,43,44 the mechanisms responsible for these effects are unknown. We propose that IAIP, through its anionic GAGs, directly binds and neutralizes the extracellular histones released during tissue damage. The increase in histone levels and the parallel decrease of IAIP during sepsis26,45 could be related. If IAIP acts as a histone scavenger and neutralizer, a decreased histone clearance as a result of IAIP downregulation could contribute to the increased levels of extracellular histone and tissue damage during sepsis progression.4,7 A similar case was described for APC, which proteolytically cleaves extracellular histones and neutralizes their cytotoxicity.7 Presence of IAIP in various plasma preparations used for resuscitation of patients with sepsis47 or trauma48 could explain their beneficial effects. Nevertheless, further work is warranted to validate this hypothesis and discern the relative contributions of histone neutralization versus protease inhibitory effects of IAIP in sepsis.
Histones are hydrophobic and cationic and could be neutralized electrostatically by anionic GAGs such as heparins.14,49 We showed that pretreatment of IAIP and HA with Polybrene abolished the protective effects on histone cytotoxicity, suggesting that an electric charge–based interaction occurs. Although both HA and the CS chain of bikunin are anionic carbohydrates, we found that HA protected the cells against histone toxicity whereas urinary bikunin did not. In contrast, pretreating IAIP with chondroitinase ABC, an enzyme that breaks down CS and to a lesser extent HA, abolished the protective effects of IAIP on histone toxicity in vitro. One explanation may be that CS chains of urinary bikunin are undersulfated50 as compared with IAIP,51 which could affect their net negative charge, or that a proportion of HC of IAIP is bound to HA, as shown by one of the authors on similar preparations of IAIP.24
Our data demonstrate that HMW-HA neutralizes histones both in vivo and in vitro. Treatment of endothelial cells with 4-MU, a potent inhibitor of HA synthesis,30 made the cells more vulnerable to histone challenge, suggesting that cell-associated HA protects against histone-induced cytotoxicity. Similarly, mutant CHO cells that lack a key enzyme of the biosynthesis pathway of all GAGs29 were more vulnerable to histones than the wild-type CHO cells, strongly supporting the protective role of cell surface glycocalyx in neutralizing extracellular histones. It is known that glycocalyx plays major protective roles in the homeostasis of the vessel wall,52 and its degradation during sepsis leads to shedding of proteoglycans and/or GAGs.53 Thus, circulating amounts of GAGs are high in patients with sepsis,54 and increased plasma levels of syndecan-154 and heparan sulfate55 have been correlated with disease severity. Our data indicate that glycocalyx shedding could be an indicator of histone scavenging by GAGs.
Besides being a component of the IAIP and the glycocalyx, HA is a polymer found in the extracellular matrix of all tissues in the body56 and has a proven anti-inflammatory role. HA is most commonly found in vivo as HMW polymers in excess of 1200 kDa. However, in inflammatory conditions, HMW-HA may undergo degradation to lower-molecular-weight forms.31 HA smaller than 500 kDa is proinflammatory through binding to CD44 receptors on leukocytes and contributing to their infiltration into inflammatory lesions, as well as inducing the expression of proinflammatory cytokines, chemokines, COX-2, iNOS, and proadhesive integrins.57-59 Here, we report that the highest inhibition of histone effects by HA correlates with its molecular weight. Our findings agree with other beneficial effects of HMW-HA, including inhibition of cytokine production, protection against T-cell–mediated liver injury,60 sepsis-induced lung injury60,61 and antiangiogenic31 effects. Detection of IAIP-histone complexes mirrored by IAIP consumption and histone and/or nucleosome release in baboon plasma during the progression of E coli sepsis supports the protective role of IAIP in sepsis. Although many broad-spectrum protease inhibitors have proven beneficial in inflammatory conditions,62,63 histone neutralization by IAIP is not dependent on the protease inhibitory moiety bikunin.
To date, beneficial effects of HA have been attributed to the blocking of lipopolysaccharide or TLRs, which mediate histone release via NETs and histone-induced inflammation.33 HMW-HA inhibits TLR2 and TLR4 signaling,31 cytokine production, leukocyte infiltration, and lung injury in sepsis.61
Since we colocalized IAIP and HA with H4 or cit-H4 in kidneys from septic baboons, particularly in the peritubular necrotic areas, we suggest that similar to heparin,6 IAIP-HA could also act directly as a trap to immobilize extracellular histones, neutralize their cytotoxicity, and counteract their organ damage effects. If confirmed in clinical samples, these data would establish that IAIP-HA-histone complexes represent a valuable biomarker for sepsis progression to organ failure.
In conclusion, our study demonstrates that anionic GAGs associated with IAIP, particularly HMW-HA, could counteract histone-induced cytotoxicity, platelet activation, and microthrombosis; prevent thrombocytopenia and prolonged bleeding time; and protect against systemic inflammation and organ injury. Because there is currently no specific treatment for sepsis, new therapies are urgently needed to effectively prevent the organ damaging effects of histones and/or nucleosomes. Direct neutralization of histones with IAIP or HA is likely to provide net advantages over APC and heparin by circumventing the bleeding complications and directly counteracting one major late-stage mediator of sepsis and sterile inflammation.
Presented in part at the American Society of Hematology Annual Meeting, New Orleans, LA, December 9, 2013.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Cristina Lupu and Mark Coggeshall for critical reading and editing of the manuscript and acknowledge the expert technical support of Joseph Qiu and Andre R. Santoso (ProThera Biologics, Inc.).
This work was supported by a grant from the National Institutes of Health, National Institute of General Medical Sciences (GM097747) (F.L.) and by institutional support from the Department of Pediatrics, University of Oklahoma Health Sciences Center (H.C.).
Authorship
Contribution: H.C. designed research, performed in vitro and animal experiments, and wrote the article; R.S.K. performed enzyme-linked immunosorbent assays and immunofluorescence staining and contributed to writing the article; R.S.-M. performed immunofluorescence staining and electron microscopy; N.I.P. performed the prothrombinase assay; P.M.-D. performed experiments and analyzed results; Y.-P.L. performed assays and provided valuable reagents; and F.L. designed research, supervised the project, and wrote the article.
Conflict-of-interest disclosure: Y.-P.L. has equity in ProThera Biologics, which is developing IAIP for commercial use. The remaining authors declare no competing financial interests.
Correspondence: Florea Lupu, Cardiovascular Biology Research Program, Oklahoma Medical Research Foundation, 825 NE 13th St, Oklahoma City, OK 73104; e-mail: florea-lupu@omrf.org.
References
Author notes
H.C. and R.S.K. contributed equally to this study.