Key Points
Entospletinib is a selective inhibitor of spleen tyrosine kinase, which is implicated in the pathobiology of B-cell lymphoid malignancies.
Entospletinib shows clinical activity in subjects with relapsed or refractory CLL with acceptable toxicity.
Abstract
Small-molecule inhibitors of kinases involved in B-cell receptor signaling are an important advance in managing lymphoid malignancies. Entospletinib (GS-9973) is an oral, selective inhibitor of spleen tyrosine kinase. This multicenter, phase 2 study enrolled subjects with relapsed or refractory chronic lymphocytic leukemia (CLL; n = 41) or non-Hodgkin lymphoma (n = 145). Participants received 800 mg entospletinib twice daily. We report efficacy outcomes in the CLL cohort (n = 41) and safety outcomes in all cohorts (N = 186). The primary end point was a progression-free survival (PFS) rate at 24 weeks in subjects with CLL. The PFS rate at 24 weeks was 70.1% (95% confidence interval [CI], 51.3%-82.7%); median PFS was 13.8 months (95% CI, 7.7 months to not reached). The objective response rate was 61.0% (95% CI, 44.5%-75.8%), including 3 subjects (7.3%) who achieved nodal response with persistent lymphocytosis. Fifty-four subjects (29.0%) had serious adverse events (SAEs). The most common treatment-emergent SAEs included dyspnea, pneumonia, febrile neutropenia, dehydration, and pyrexia. Common grade 3/4 laboratory abnormalities included neutropenia (14.5%) and reversible alanine aminotransferase/aspartate aminotransferase elevations (13.4%). Entospletinib demonstrates clinical activity in subjects with relapsed or refractory CLL with acceptable toxicity. This trial was registered at www.clinicaltrials.gov as #NCT01799889.
Introduction
Spleen tyrosine kinase (Syk) is a cytoplasmic protein tyrosine kinase that is predominantly expressed in cells of hematopoietic lineage. Syk functions normally to couple activated immunoreceptors to downstream signaling pathways. Syk signaling elicits a range of diverse biologic functions, including cellular development, function, proliferation, differentiation, and adhesion.
In a normal, resting B cell, Syk is unphosphorylated and inactive.1 On antigen stimulation of the B-cell receptor (BCR), Syk is recruited to the immunoreceptor, tyrosine-based activation motifs of cluster of differentiation (CD) 79a/b, where it undergoes phosphorylation and activation.2,3 Phosphorylation of specific tyrosine residues within the Syk protein creates docking sites for signaling protein substrates, including B-cell linker protein, phosphatidylinositol 3-kinase, protein kinase B, phospholipase C γ 2, and extracellular signal–regulated kinases, thus activating BCR pathway signals.4
Constitutive activation of Syk and the BCR pathway has been demonstrated to be essential for cell proliferation and survival in multiple B-cell malignancies.5-7 In subjects with chronic lymphocytic lymphoma (CLL), malignant cells continuously circulate between secondary lymphoid organs, where cells undergo proliferation, and the peripheral circulation, where anergic cells recover their proliferative potential. This balance is mediated by the capacity of CLL cells to signal through the BCR signaling pathway and to control the resulting cytokine production and signaling cascade.7
In CLL cells, BCR stimulation results in production of the chemokines CCL3 and CCL4, which are found at high levels in subjects with CLL. These chemokines and others result in the sequestration of malignant CLL cells within secondary lymphoid tissues and facilitate important cell–cell interactions with stromal components that promote their survival in vivo.8 In addition, CLL cell chemotaxis toward CXCL12 and CXCL13 and retention within lymphoid tissues depend on signaling through Syk.9,10 These findings support the hypothesis that Syk inhibitors can release malignant B cells from microenvironmental niches in the lymph nodes and limit their homing, residence, and proliferation in these protected environments.
Clinical investigation of fostamatinib, the prodrug of the Syk inhibitor R406, in a phase 2 trial in B-cell malignancies demonstrated a 55% nodal response rate in CLL/small lymphocytic lymphoma at 200 or 250 mg twice a day (BID).11 Toxicities reported included diarrhea, nausea, hypertension, cytopenias, and fatigue, which limited dosing and have been partially attributed to off-target effects, including the inhibition of kinases in addition to Syk. Further clinical investigation of this agent in B-cell malignancies has not been reported.
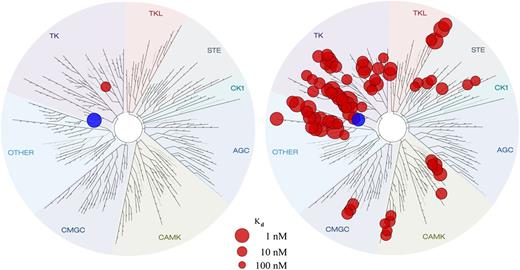
Entospletinib (GS-9973) is an adenosine triphosphate competitive inhibitor of Syk that disrupts kinase activity with a half maximal inhibitory concentration of 7.6 nM (supplemental Figure 1, available on the Blood Web site). Broad kinase panel screening revealed a greater selectivity of entospletinib vs R406.12 Dissociation constant (Kd) determinations of the strongest hits from the broad panel KINOMEscan (DiscoveRx Corporation, San Diego, CA) showed that aside from Syk itself, only 1 kinase, TNK1, had a Kd < 100 nM for entospletinib, whereas 79 kinases had Kd < 100 nM for R406 (Figure 1). In healthy volunteers, entospletinib at BID doses higher than 200 mg demonstrated inhibition of Syk activity, as measured by CD63 expression and phospho-Syk.13 Because of its excellent selectivity profile, entospletinib was hypothesized to provide high levels of Syk inhibition with potentially fewer off-target adverse effects (AEs) than observed with fostamatinib.12
Entospletinib shows greater selectivity for Syk than for R406. The blue circle represents Syk. Kinase activity for entospletinib is illustrated on the left, and kinase activity for R406 is illustrated on the right. Entospletinib is Syk-selective (Syk Kd = 7.6 nM, with only 1 other kinase with a Kd < 100 nM). R406 is nonselective (Syk Kd = 15 nM, with 25 kinases with Kd < 15 nM and 54 additional kinases with Kd < 100 nM).
Entospletinib shows greater selectivity for Syk than for R406. The blue circle represents Syk. Kinase activity for entospletinib is illustrated on the left, and kinase activity for R406 is illustrated on the right. Entospletinib is Syk-selective (Syk Kd = 7.6 nM, with only 1 other kinase with a Kd < 100 nM). R406 is nonselective (Syk Kd = 15 nM, with 25 kinases with Kd < 15 nM and 54 additional kinases with Kd < 100 nM).
In this phase 2 trial, the safety and efficacy of entospletinib was tested in separate cohorts of subjects with CLL, indolent non-Hodgkin lymphoma, mantle cell lymphoma (MCL), or diffuse large B-cell lymphoma (DLBCL). In this article, we report the efficacy of entospletinib in the cohort of subjects with relapsed or refractory CLL and the initial safety results from all patients enrolled in the study.
Methods
Study design and conduct
We performed a phase 2, open-label, single-agent study evaluating the efficacy, safety, tolerability, and pharmacodynamics of entospletinib in subjects with relapsed or refractory hematologic malignancies. Five separate cohorts consisting of subjects with CLL, follicular lymphoma (FL), other indolent non-Hodgkin lymphomas (including lymphoplasmacytic lymphoma, small lymphocytic lymphoma, and marginal zone lymphoma), MCL, or DLBCL were enrolled. A Bayesian, continuous data review approach was used to update the estimates of progression-free survival (PFS) rates at 16 (MCL and DLBCL) or 24 (CLL and FL) weeks and to assess futility with a maximum of 40 subjects per cohort. The cohort of other indolent non-Hodgkin lymphomas (excluding FL) continues to enroll 40 subjects without futility assessment. The study was conducted at multiple centers in North America and was registered at clinicaltrials.gov as NCT01799889. The study protocol, amendments, informed consent according to the Declaration of Helsinki, and other information that required preapproval were approved by the relevant institutional review boards.
Eligibility
Subjects who were at least 18 years of age with a documented diagnosis of CLL with progressive disease and who met the criteria for treatment by the International Workshop on CLL were eligible for the study. Prior treatment of CLL must have included either a regimen containing a therapeutic antibody administered for 2 or more doses of antibody treatment or a regimen containing at least 1 cytotoxic agent administered for 2 or more cycles of cytotoxic treatment. The presence of radiographically measurable lymphadenopathy or extranodal lymphoid malignancy as assessed by computed tomography or magnetic resonance imaging was required for enrollment. All acute toxic effects of prior antitumor therapy must have resolved to grade 1 or lower before the start of the study drug, with the exception of alopecia (grade 1 or 2 permitted), neurotoxicity (grade 1 or 2 permitted), or bone marrow parameters (grade 1, 2, or 3 permitted). A Karnofsky performance status of at least 60% and a life expectancy of at least 3 months were required. Required screening laboratory data were collected within 5 weeks before administration of the study drug. Subjects with any degree of neutropenia, thrombocytopenia, or anemia attributed to the malignancy by the treating physician were eligible to enroll.
Subjects were not eligible if they met any of the following criteria: known active central nervous system lymphoma; intermediate- or high-grade myelodysplastic syndrome; history of a nonlymphoid malignancy, with limited exceptions; evidence of an ongoing systemic infection; pregnant or breastfeeding; history or prior allogeneic bone marrow progenitor cell or solid organ transplantation; or receipt of investigational medication within 21 days of study entry. Concurrent therapy with agents that reduce gastric acidity was not allowed because of the anticipated reduction of entospletinib absorption.
Treatment
Treatment with entospletinib was at a dose of 800 mg BID over the course of 28-day cycles under fasting conditions. Fasting was defined as no food or liquids other than water for 2 hours predose and 1 hour postdose.
Assessments
Clinic visits, including laboratory tests and pharmacodynamic measurements (including serum cytokines and protein phosphorylation in circulating CLL cells) were scheduled for day 1 of each 28-day cycle. Blood samples for pharmacokinetic analyses of entospletinib were collected on day 1 of each 28-day cycle; on days 8, 15, and 22 of cycle 1; and on day 15 of cycle 2. Additional safety monitoring visits took place on days 8, 15, and 22 of cycle 1 and day 15 of cycle 2.
Procedures to assess tumor response were conducted every 8 weeks during the first 24 weeks on study and then every 12 weeks thereafter until disease progression or the start of other antitumor therapies, regardless of cycle number or dose interruptions. Computed tomography or magnetic resonance imaging scans were used to document sites of disease, identify target lesions, and assess response and disease progression. Determination of response and progression was based on standardized International Workshop on CLL criteria14 in the planned protocol analysis. In this reporting, we are using the updated workshop criteria modified by Cheson et al.15 These criteria state that a lymph node response with persistent lymphocytosis does not change the designation of partial response (PR). The findings of an Independent Review Committee were used for analyses of PFS and other tumor control end points.
It was recommended that a bone marrow biopsy and aspirate be collected for confirmatory purposes for all subjects who achieved a radiologic complete response (CR) or to confirm suspected progressive disease based solely on declines in the platelet count and/or hemoglobin.
End points
The primary end point was the PFS rate at 24 weeks for subjects with CLL. Secondary end points included tolerability, objective response rate (ORR), duration of response (DOR), time to response, and lymph node response rate. Safety assessments, including all abnormal laboratory data and AEs, were performed by grading the laboratory values and AEs according to the Common Terminology Criteria for AEs version 4.03.
Exploratory end points included plasma drug concentrations and pharmacodynamic biomarkers.
Statistical analyses
A Bayesian, continuous data review approach was used to update the estimates of PFS rates at 16 weeks (MCL and DLBCL) or 24 weeks (CLL and FL) and to assess futility with a maximum size of 40 subjects per cohort. The cohort was considered to have crossed the futility boundary if it were highly likely (>90%) that the PFS rate was less than 0.2, given the available efficacy outcomes data at 16 or 24 weeks.
PFS was defined as the interval from start of treatment to disease progression or death from any cause, analyzed using Kaplan-Meier methods. The findings of the Independent Review Committee were considered primary for analyses of PFS and other tumor control responses. The ORR was defined as the proportion of subjects who had a CR or PR. DOR was defined as the time from when the first response (CR or PR) was achieved until the earlier of the first documentation of definitive disease progression or death from any cause. The lymph node response rate was defined as the proportion of subjects who had a decrease of 50% or more in lymphadenopathy, irrespective of lymphocyte count.
Results
Subjects
Forty-one subjects with CLL were enrolled in the study between April 24, 2013, and October 21, 2013, and were included for the efficacy analysis. One hundred eighty-six subjects with CLL or NHL were enrolled between March 20, 2013, and November 3, 2014, and were included for the safety analysis. The data cutoff for this analysis was November 6, 2014. For the 41 CLL subjects, the median age was 73 years (range, 51-89 years), 68% were male, and the median number of prior treatment regimens was 2 (range, 1-8). Prior treatments included anti-CD20 antibodies (97.6%), alkylating agents (85.4%; bendamustine [63.4%]), and fludarabine (68.3%). Ten subjects (24.4%) had 17p deletions or TP53 mutations, 10 subjects (24.4%) had 11q deletions, and 7 subjects (17.1%) had NOTCH1 or SF3B1 mutations. Twelve subjects (29.3%) did not have any of these mutations or deletions, and the genetic status of 2 subjects (4.9%) was undetermined. Eight of 39 subjects (19.5%) were immunoglobulin heavy chain variable region gene (IGHV) mutated, 31 subjects (75.6%) were IGHV unmutated, and mutational status was undetermined in 2 subjects (4.9%). The most common reasons for discontinuation of study drug were progressive disease and AEs (Table 1).
Characteristics of the subjects with CLL at baseline and study status (N = 41)
| Characteristic . | . |
|---|---|
| Median age (range), year | 73 (51-89) |
| Rai stage (% of subjects) | |
| 0 | 3 (7.3%) |
| 1 or 2 | 18 (43.9%) |
| 3 or 4 | 20 (48.8%) |
| Extent of CLL (% of subjects) | |
| Anemia | |
| Any grade | 21 (51.2%) |
| Grade ≥ 3 | 0 |
| Neutropenia | |
| Any grade | 14 (34.1%) |
| Grade ≥ 3 | 2 (4.9%) |
| Thrombocytopenia | |
| Any grade | 22 (53.7%) |
| Grade ≥ 3 | 1 (2.4%) |
| Median ALC (range), mm3 | 30 070 (740-222 200) |
| Median estimated creatinine clearance (range), mL/minute | 63.8 (25.2-123.6) |
| Genetic risk factors from high to low risk (% of subjects) | |
| 17p deletion or TP53 mutation | 10 (24.4%) |
| 11q deletion | 10 (24.4%) |
| NOTCH1 or SF3B1 mutation | 7 (17.1%) |
| None of the above mutations or deletions | 12 (29.3%) |
| Undetermined | 2 (4.9%) |
| IGHV status | |
| Mutated | 8 (19.5%) |
| Unmutated | 31 (75.6%) |
| Undetermined | 2 (4.9%) |
| Previous CLL treatment | |
| Median number of regimens (range) | 2 (1-8) |
| Drugs (% of subjects) | |
| Anti-CD20 agents | 40 (97.6%) |
| Rituximab | 39 (95.1%) |
| Ofatumumab | 5 (12.2%) |
| Alkylating agents | 35 (85.4%) |
| Bendamustine | 26 (63.4%) |
| Fludarabine | 28 (68.3%) |
| Study status (%) | |
| Treated | 41 |
| Continued study drug | 16 (39.0%) |
| Discontinued study drug | 25 (61.0%) |
| Adverse events | 4 (8.8%) |
| Progressive disease | 16 (39.0%) |
| Death | 1 (2.4%) |
| Investigator decision | 3 (7.3%) |
| Withdrawn consent | 1 (2.4%) |
| Discontinued study | 25 (61%) |
| Characteristic . | . |
|---|---|
| Median age (range), year | 73 (51-89) |
| Rai stage (% of subjects) | |
| 0 | 3 (7.3%) |
| 1 or 2 | 18 (43.9%) |
| 3 or 4 | 20 (48.8%) |
| Extent of CLL (% of subjects) | |
| Anemia | |
| Any grade | 21 (51.2%) |
| Grade ≥ 3 | 0 |
| Neutropenia | |
| Any grade | 14 (34.1%) |
| Grade ≥ 3 | 2 (4.9%) |
| Thrombocytopenia | |
| Any grade | 22 (53.7%) |
| Grade ≥ 3 | 1 (2.4%) |
| Median ALC (range), mm3 | 30 070 (740-222 200) |
| Median estimated creatinine clearance (range), mL/minute | 63.8 (25.2-123.6) |
| Genetic risk factors from high to low risk (% of subjects) | |
| 17p deletion or TP53 mutation | 10 (24.4%) |
| 11q deletion | 10 (24.4%) |
| NOTCH1 or SF3B1 mutation | 7 (17.1%) |
| None of the above mutations or deletions | 12 (29.3%) |
| Undetermined | 2 (4.9%) |
| IGHV status | |
| Mutated | 8 (19.5%) |
| Unmutated | 31 (75.6%) |
| Undetermined | 2 (4.9%) |
| Previous CLL treatment | |
| Median number of regimens (range) | 2 (1-8) |
| Drugs (% of subjects) | |
| Anti-CD20 agents | 40 (97.6%) |
| Rituximab | 39 (95.1%) |
| Ofatumumab | 5 (12.2%) |
| Alkylating agents | 35 (85.4%) |
| Bendamustine | 26 (63.4%) |
| Fludarabine | 28 (68.3%) |
| Study status (%) | |
| Treated | 41 |
| Continued study drug | 16 (39.0%) |
| Discontinued study drug | 25 (61.0%) |
| Adverse events | 4 (8.8%) |
| Progressive disease | 16 (39.0%) |
| Death | 1 (2.4%) |
| Investigator decision | 3 (7.3%) |
| Withdrawn consent | 1 (2.4%) |
| Discontinued study | 25 (61%) |
ALC, absolute lymphocyte count; IGHV, immunoglobulin heavy chain variable.
Receipt of study drug
At the time of this analysis, the median time that CLL subjects had received entospletinib was 36 weeks (interquartile range, 15-67 weeks; simple range, 1-80 weeks), and 22 subjects (53.7%) received entospletinib for 6 months or longer. Nine subjects (22.0%) required at least 1 dose reduction from the starting dose, 7 subjects (17.1%) had their dose reduced to 600 mg, and 2 subjects (4.9%) had the dose reduced to 400 mg. Three subjects in the CLL group had treatment-emergent AEs leading to dose reduction. These AEs included grade 3 alanine aminotransferase (ALT)/aspartate aminotransferase (AST) elevations, grade 2 fatigue, grade 2 cognitive disorders, and grade 2 muscular weakness. Sixteen CLL subjects are still receiving treatment. The most common reason for discontinuation of study treatment was disease progression.
Efficacy
PFS.
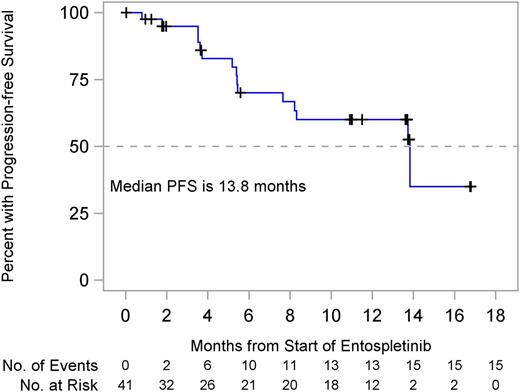
The CLL cohort enrolled 41 subjects without crossing the futility boundary with a median follow-up of 7.7 months. The primary end point of 24 weeks PFS was 70.1% (95% confidence interval [CI], 51.3%-82.7%). Median PFS was 13.8 months (95% CI, 7.7 months to not reached; Figure 2). There were 15 subjects (36.6%) with events, 14 (34.1%) with disease progression, and 1 death (2.4%) attributed to septic pneumonia unrelated to entospletinib.
ORR in CLL subjects.
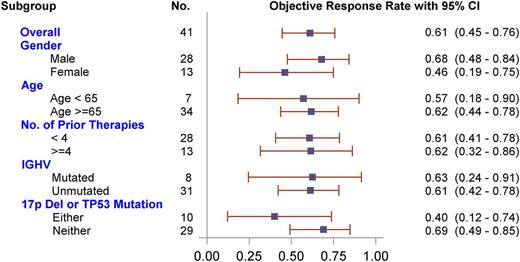
The ORR was 61.0% (95% CI, 44.5%-75.8%), with 25 subjects achieving a PR and no subject achieving a CR. Three of the patients (7.3%) achieved nodal response (50% reduction in the sum of the products of the diameters [SPD]) with persistent lymphocytosis. Thirteen subjects (31.7%) had stable disease. One subject (2.4%) had progressive disease; 1 subject (2.4%) had an unscheduled scan before death resulting from pneumonia unrelated to study drug, but the scan was not sufficient for evaluation; and 1 subject (2.4%) withdrew consent before the first scheduled assessment. There was no significant difference in ORR within subgroups, including sex, age, and number of prior therapies, IGHV mutation status, or 17p deletion/TP53 mutation status (Figure 3), although a statistically insignificant trend toward lower response rate was seen in the 17p deletion/TP53 mutation. Sixteen of 25 responding subjects (64.0%) had a PR at the first postbaseline evaluation (scheduled at week 8).
DOR in CLL subjects.
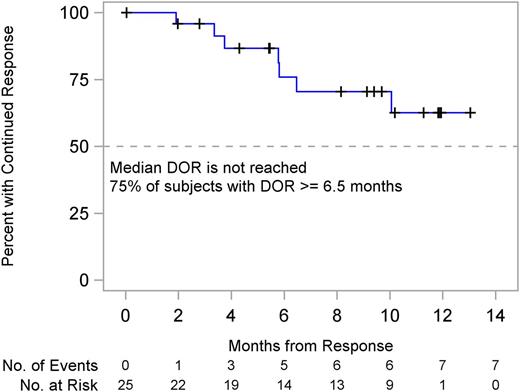
Among the 25 responding subjects, median DOR had not yet been reached (95% CI, 6.5 months to not reached). Seventy-five percent of subjects have had a DOR not less than 6.5 months (95% CI, 1.9 months to not reached) (Figure 4).
Lymph node response in subjects with CLL.
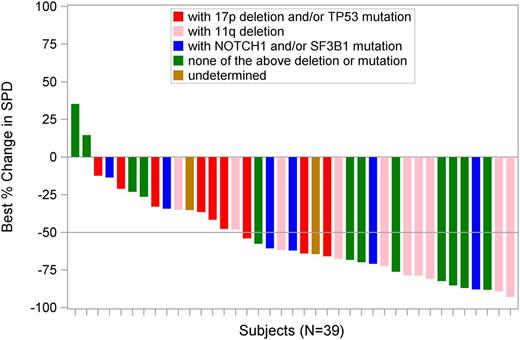
The best percentage changes in the SPD of measured lymph nodes, as determined by central review, are illustrated in Figure 5. Of 39 evaluable subjects who had at least 1 postbaseline assessment, 94.9% achieved a reduction in adenopathy. At least a 50% decrease in SPD from baseline was achieved by 61.5% (95% CI, 44.6%-76.6%) of subjects, and 33% had a less than 50% decrease in SPD from baseline.
Changes in the measured size of lymph nodes from baseline in subjects with CLL (N = 39).
Changes in the measured size of lymph nodes from baseline in subjects with CLL (N = 39).
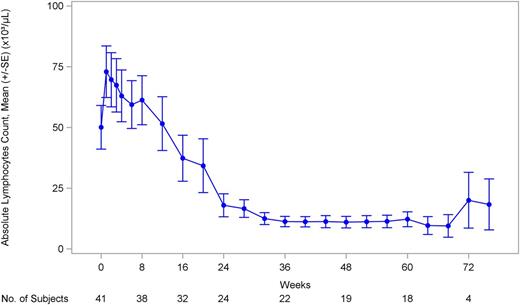
Of 41 CLL subjects, 36 had abnormal absolute lymphocyte counts (ALCs; ≥4 × 109/L) at baseline. Among the subjects with abnormal ALCs at baseline, 20 experienced a 50% or more reduction in ALC, or ALC returned to normal levels (<4 × 109/L). The median time to reach a 50% or more reduction or normal level was 16.2 weeks. The mean ALC in subjects rose from 50 090 to 72 980 lymphocytes per microliter on day 8 and then declined (Figure 6), returning to baseline by approximately 3 months, and then declining further.
Safety
The safety analysis included all treated subjects (N = 186) in each cohort of the study, including those with CLL, FL, lymphoplasmacytic lymphoma/small lymphocytic lymphoma/marginal zone lymphoma, MCL, or DLBCL. Of all treated subjects, 96.8% had at least 1 treatment-emergent AE. Treatment-emergent, nonhematologic AEs that occurred in 15% or higher at any grade in all subjects, serious AEs (SAEs) that occurred in at least 2%, and common laboratory abnormalities are reported in Table 2. The most common AEs were fatigue and gastrointestinal disturbances. Among 147 subjects who experienced entospletinib-related, treatment-emergent AEs, 77 (52.4%) had entospletinib-related treatment-emergent AEs that were grade 2 or lower.
Treatment-emergent AEs and laboratory abnormalities (N = 186)
| . | Any grade, n (%) . | At least grade 3, n (%) . |
|---|---|---|
| AE (at least 15% nonhematologic AEs in any grade) | ||
| Fatigue | 99 (53.2) | 19 (10.2) |
| Nausea | 84 (45.2) | 7 (3.8) |
| Diarrhea | 72 (38.7) | 3 (1.6) |
| Decreased appetite | 46 (24.7) | 2 (1.1) |
| Constipation | 44 (23.7) | 3 (1.6) |
| Cough | 39 (21) | 1 (0.5) |
| Headache | 39 (21) | 1 (0.5) |
| Dizziness | 37 (19.9) | 1 (0.5) |
| Pyrexia | 36 (19.4) | 3 (1.6) |
| Vomiting | 34 (18.3) | 2 (1.1) |
| Dyspnea | 29 (15.6) | 12 (6.5) |
| Insomnia | 29 (15.6) | 0 (0) |
| SAE (at least 2%) | ||
| Dyspnea | 8 (4.3) | |
| Pneumonia | 8 (4.3) | |
| Febrile neutropenia | 6 (3.2) | |
| Dehydration | 5 (2.7) | |
| Pyrexia | 4 (2.2) | |
| Laboratory abnormality | ||
| Neutropenia | 61 (32.8) | 27 (14.5) |
| Anemia | 71 (38.2) | 15 (8.1) |
| Thrombocytopenia | 16 (8.6) | 5 (2.7) |
| Total bilirubin | 60 (32.3) | 7 (3.8) |
| Increased ALT | 68 (36.6) | 24 (12.9) |
| Increased AST | 61 (32.8) | 19 (10.2) |
| . | Any grade, n (%) . | At least grade 3, n (%) . |
|---|---|---|
| AE (at least 15% nonhematologic AEs in any grade) | ||
| Fatigue | 99 (53.2) | 19 (10.2) |
| Nausea | 84 (45.2) | 7 (3.8) |
| Diarrhea | 72 (38.7) | 3 (1.6) |
| Decreased appetite | 46 (24.7) | 2 (1.1) |
| Constipation | 44 (23.7) | 3 (1.6) |
| Cough | 39 (21) | 1 (0.5) |
| Headache | 39 (21) | 1 (0.5) |
| Dizziness | 37 (19.9) | 1 (0.5) |
| Pyrexia | 36 (19.4) | 3 (1.6) |
| Vomiting | 34 (18.3) | 2 (1.1) |
| Dyspnea | 29 (15.6) | 12 (6.5) |
| Insomnia | 29 (15.6) | 0 (0) |
| SAE (at least 2%) | ||
| Dyspnea | 8 (4.3) | |
| Pneumonia | 8 (4.3) | |
| Febrile neutropenia | 6 (3.2) | |
| Dehydration | 5 (2.7) | |
| Pyrexia | 4 (2.2) | |
| Laboratory abnormality | ||
| Neutropenia | 61 (32.8) | 27 (14.5) |
| Anemia | 71 (38.2) | 15 (8.1) |
| Thrombocytopenia | 16 (8.6) | 5 (2.7) |
| Total bilirubin | 60 (32.3) | 7 (3.8) |
| Increased ALT | 68 (36.6) | 24 (12.9) |
| Increased AST | 61 (32.8) | 19 (10.2) |
Grade 3 fatigue was reported in 19 subjects (10.2%), grade 3 nausea was reported in 7 subjects (3.8%), and grade 3 dyspnea was reported in 12 subjects (6.5%). One subject (0.5%) experienced a grade 4 rash, 5 subjects (2.7%) had grade 3 rashes, and 4 subjects (2.2%) had grade 2 rashes.
Fifty-four subjects (29.0%) experienced treatment-emergent SAEs, the most common of which included dyspnea (n = 8), pneumonia (n = 8), febrile neutropenia (n = 6), dehydration (n = 5), and pyrexia (n = 4). Of 41 subjects with CLL, 1 withdrew because of an SAE of hepatotoxicity. Four subjects with CLL discontinued study drug because of an AE; before discontinuation, the best response for these 4 subjects was stable disease (n = 2) and PR (n = 2). Twenty-nine subjects (15.6%) experienced a treatment-emergent AE that led to study drug discontinuation, including fatigue (n = 4), increased ALT (n = 3), and headache (n = 3).
Common laboratory abnormalities reported for any subjects enrolled in the trial are listed in Table 2. Subjects were allowed to enroll in the study if they had cytopenias resulting from CLL. Treatment-emergent cytopenias were defined as a cytopenia that worsened in the period from the first dose of study treatment to 30 days after the last dose of study treatment. Grade 3 or higher neutropenia was reported in 27 subjects (14.5%), and grade 3 or higher anemia was reported in 15 subjects (8.1%). Reversible grade 3 or 4 ALT/AST elevations occurred in 25 of 186 subjects (13.4%), 13 of which occurred by 6 weeks (first and third quartile of 2.1 and 6.1 weeks, respectively), and all but 2 of which occurred by 10 weeks. Treatment was interrupted and resumed in 14 (56.0%) of 25 subjects without further event. One subject continued on 800 mg BID without further event, 7 discontinued for other reasons while entospletinib was being withheld, 1 experienced grade 3 ALT/AST elevation at disease progression and then discontinued treatment, and 2 withdrew because of ALT/AST elevation. (One subject experienced a grade 4 ALT/AST elevation on day 29; entospletinib was withdrawn on day 30, and both ALT and AST resolved to grade 1 on day 58. The other subject experienced a grade 3 ALT elevation on day 43, entospletinib was withdrawn on day 44, and both ALT and AST resolved to grade 1 or lower on day 56.)
There were 23 deaths in the study: 19 resulting from disease progression and 4 resulting from AEs. The 4 AEs leading to death while on study include respiratory failure (n = 1), sepsis (n = 2), and bowel obstruction (n = 1). None of the AEs resulting in death was attributed to entospletinib by investigators.
Pharmacokinetics and pharmacodynamics
The mean (percentage coefficient of variation) entospletinib plasma pharmacokinetic exposure parameters were as follows: maximum plasma concentration, 1490 (38%) ng/mL; plasma concentration–time curve for a dosing interval, 15 500 (40%) ng/hour/mL; and minimum plasma concentration, 1100 (44%) ng/mL. Overall, the entospletinib minimum plasma concentration was well above the in vitro, whole-blood, half-maximal response concentration for Syk inhibition (314 ng/mL).
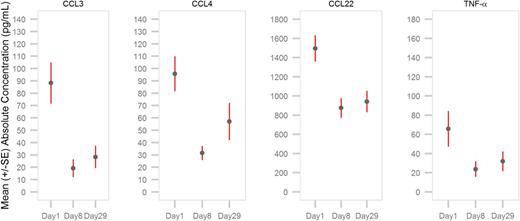
As illustrated in Figure 7, significant reductions in the serum levels of BCR-induced CCL3 and CCL4 chemokines were observed in subjects at first evaluation on days 8 and 29 of treatment (nominal P < .0001). Serum levels of CCL22 and tumor necrosis factor α were also inhibited on days 8 and 29 of therapy (nominal P < .0001).
Inhibition of chemokines and cytokines in subjects with CLL after treatment with entospletinib. Serum levels of BCR-mediated chemokines/cytokines, including CCL3/MIP-1α, CCL4/MIP-1β, CCL22/MDC, and tumor necrosis factor α, were measured on day 1 before entospletinib treatment and on days 8 and 29 by Luminex (Life Technologies/Thermo Fisher Scientific, Waltham, MA) immunoassays (n = 40). Entospletinib reduced mean plasma levels of all measured chemokines/cytokines over time (nominal P < .0001).
Inhibition of chemokines and cytokines in subjects with CLL after treatment with entospletinib. Serum levels of BCR-mediated chemokines/cytokines, including CCL3/MIP-1α, CCL4/MIP-1β, CCL22/MDC, and tumor necrosis factor α, were measured on day 1 before entospletinib treatment and on days 8 and 29 by Luminex (Life Technologies/Thermo Fisher Scientific, Waltham, MA) immunoassays (n = 40). Entospletinib reduced mean plasma levels of all measured chemokines/cytokines over time (nominal P < .0001).
Discussion
Novel agents targeting the BCR signaling cascade are transforming the management of patients with CLL. Inhibition of key signaling enzymes within the pathway, including Syk, Bruton’s tyrosine kinase, and phosphatidylinositol 3-kinase δ, is becoming an important therapeutic strategy.
The first agent to demonstrate the therapeutic potential of BCR signaling inhibition was fostamatinib. In the phase 2 component of a multicenter study evaluating patients with a variety of B-cell malignancies, 6 (54%) of 11 patients with CLL experienced a reduction in lymph node size greater than 50%, and a median PFS of 6.4 months was reported. Updated response criteria accounting for lymphocytosis were not available at the time of the report, and the lymphocytosis characteristics were not described.11
In this current study of entospletinib, 39 (95.1%) of 41 patients were evaluable for lymph node response. Of evaluable patients, 94.5% experienced some reduction in adenopathy, and 24 (61.5%) of 39 patients experienced lymph node reductions of at least 50%. Twenty-five (61.0%) of 41 patients achieved a PR (no patient achieved a CR). Reported rates of AEs were similar between the 2 agents, with more hypertension resulting from fostamatinib and more frequent liver function test abnormalities seen for patients treated with entospletinib. In general, entospletinib was well tolerated by most patients; the most common reason for drug discontinuation was disease progression (16/25), rather than AEs.
The efficacy of ibrutinib monotherapy in relapsed CLL has been reported in 2 studies. In a randomized phase 3 study of ibrutinib versus ofatumumab, the response rate to ibrutinib was 43% at a median follow-up of 9.4 months,16 whereas the response rate to ibrutinib in a single-arm, phase 2 study was 71% at a median follow-up of 20.9 months.17 The difference in response rates in these 2 studies can be explained in part by the duration of follow-up, as response rates to BCR signaling inhibitors appear to improve with longer exposures to therapy.17 This report of entospletinib describes the safety and response characteristics based on a median follow-up of 7.7 months. Response rates may change with longer follow-up, and additional CLL cohorts are currently being accrued, which will be reported with longer exposure to therapy.
Median PFS for ibrutinib has not yet been reported; however, the 12-month PFS is approximately 70% to 80%16,17 vs 60% for entospletinib in the current study. Those patients who experience early progression when receiving ibrutinib or who are intolerant of the medication are likely to need alternative treatment options. The activity of entospletinib in patients exposed to prior ibrutinib or idelalisib has not yet been defined. However, accrual of such patients is ongoing.
Idelalisib was approved in combination with rituximab in patients with relapsed CLL18 ; however, the single-agent experience was recently reported.19 In this phase 1 study with expansion cohorts, monotherapy with idelalisib resulted in an overall response rate of 72%, with 39% achieving a PR and 33% achieving a PR with residual lymphocytosis.19 The median PFS for idelalisib monotherapy was 15.8 months, although there was a significant difference between patient outcomes based on idelalisib dose level, which favored higher doses.19 In the present study, entospletinib had a median PFS of 13.8 months. Among entospletinib-treated patients who achieved a PR, those responses have been very durable, with 75% of patients remaining free from progression at 6.5 months. Whether entospletinib dose level affects outcome is currently being evaluated with a novel pill formulation designed to overcome the decreased absorption with higher gastric pH, and additional patients are being enrolled in a dose-optimization study.
It is unclear whether the optimal role for entospletinib will be as monotherapy or in combination with other agents. Although the favorable hematopoietic toxicity profile suggests entospletinib may be appropriate in combination with other agents, future studies will be required to determine whether the best combination therapies are other agents targeting BCR signaling or other pathways, such as BCL-2, or even standard chemoimmunotherapy regimens. The initial attempts to combine entospletinib with idelalisib resulted in an unexpected pneumonitis syndrome attributed to excessive mammalian target of rapamycin–like activity of the combination at the dose and schedule studied.20
Therapeutic inhibition of kinases involved within the BCR signaling cascade is an emerging field, and many questions remain. The activity of multiple, small-molecule inhibitors targeting different enzymes within the pathway creates significant questions regarding the optimal sequencing of agents, combination therapy, and management after development of resistance to a specific agent. The efficacy and tolerability of entospletinib in this population suggest there may be a suitable role for this agent in the management of patients with CLL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Julie Di Paolo, Srini Ramanathan, and Anita Reddy for assistance with the collection and analysis of data and Stephanie Kushner for editorial assistance in preparing the manuscript.
Financial support for this study was provided by Gilead Sciences, Inc.
Authorship
Contribution: J.S. designed and performed research, analyzed data, and wrote the paper; M.H. designed research and analyzed data; M.B., L.K., K.K., and C.Y. performed research and analyzed data; and S.A., J.H., and M.W. analyzed data and wrote the manuscript.
Conflict-of-interest disclosure: J.S. received research funding and honoraria from Gilead Sciences, Inc.; Pharmacyclics Inc.; and Genentech, Inc., and received research funding and consulting fees from Celgene Corporation. K.K. received research funding from Gilead Sciences, Inc.; Genentech, Inc.; TG Therapeutics, Inc.; GlaxoSmithKline; Janssen Pharmaceuticals, Inc.; and Pharmacyclics, Inc. M.H., M.W., J.H., and S.A. own stock in Gilead Sciences, Inc. C.Y. received research funding from Gilead Sciences, Inc. The remaining authors declare no competing financial interests.
Correspondence: Jeff Sharman, Willamette Valley Cancer Institute, 3377 RiverBend Drive, Suite 500, Springfield, OR 97477; e-mail: jeff.sharman@usoncology.com.